Abstract
The immune system establishes during the prenatal period from distinct waves of stem and progenitor cells and continuously adapts to the needs and challenges of early postnatal and adult life. Fetal immune development not only lays the foundation for postnatal immunity but establishes functional populations of tissue-resident immune cells that are instrumental for fetal immune responses amidst organ growth and maturation. This review aims to discuss current knowledge about the development and function of tissue-resident immune populations during fetal life, focusing on the brain, lung, and gastrointestinal tract as sites with distinct developmental trajectories. While recent progress using system-level approaches has shed light on the fetal immune landscape, further work is required to describe precise roles of prenatal immune populations and their migration and adaptation to respective organ environments. Defining points of prenatal susceptibility to environmental challenges will support the search for potential therapeutic targets to positively impact postnatal health.
Keywords: Fetal immunity, Prenatal development, Embryogenesis, Immune ontogeny
Introduction
The development of the immune system during the prenatal period is layered in time and space to meet developmental needs and prepare the human fetus for postnatal life [1]. The identification of fetal immune cell populations specific to their tissue environment at a certain fetal age suggests that the immune system serves roles for organ growth and maturation [2–5]. Furthermore, strong evidence describes the persistence and self-maintenance of fetal-derived immune cell populations in most adult tissues, contributing to the resident immune compartments independent of adult long-term hematopoietic stem cells (HSC) [6, 7]. This exemplifies the potential for lasting impact of the prenatal period on postnatal immunity. Tissue residency is conventionally assigned to an immune population that possesses self-renewal capacity, expresses a tissue-specific transcriptional program, e.g., homing receptors, and undergoes little to no exchange with the circulating pool of immune cells [8, 9]. During embryogenesis, the spatiotemporal seeding of the immune system in the various organ systems can be considered the establishment of immune tissue residency. We here review the formation of tissue-resident immunity in the human fetus, supported by evidence from animal models, focusing on recent insights in prenatal gut, lung, and brain development.
Establishment of the fetal immune system in time and space
The immune system develops in spatiotemporarily highly coordinated waves of hematopoiesis [1, 10], described for mouse myeloid lineages as primitive, transient definitive, and definitive waves [11]. In humans, early hematopoietic cells (primitive wave) are detected in the extraembryonic yolk sac (YS) around 4 postconception weeks (PCW1). Primitive HSC-like and erythro-myeloid progenitors (EMPs) give rise to premacrophages, mast cells, natural killer (NK), and innate lymphoid cell (ILC) progenitors, while megakaryocytes and erythroid cells enable oxygen supply to the growing fetus [12–14]. Concomitantly, definitive HSC and multipotent progenitors (MPP) that are capable to give rise to all mature blood and immune cells for the life of the individual are generated in the intraembryonic hemogenic endothelium in the aorta-gonad-mesonephros (AGM) region [15–17] and other hemogenic regions, including the umbilical cord and placenta [18]. In human fetuses after 6 PCW, YS- and AGM-derived macrophages and mast cells can be detected among fetal peripheral tissues such as skin, brain, and kidney; some of them constitute lifelong self-maintained populations that are independent from repopulation by bone marrow (BM)–derived precursors, as shown in mice, while others such as in the gut will be gradually replaced by circulating precursors derived from definitive HSC [11, 12, 14, 15, 19]. Between 6–9 PCW, both YS- and AGM-derived progenitors and definitive HSC with long-term repopulation capacity will seed the fetal liver to commence definitive hematopoiesis with long-term reconstitution potential [20, 21]. The fetal liver is the major hematopoietic organ during fetal development, contributing during all developmental stages and supporting active erythro-myeloid hematopoiesis and HSC expansion [12, 22]. After establishment of the hematopoietic fetal liver, the fetal BM will be seeded by fetal liver–derived HSC at 11–12 PCW, establishing it as the niche for lifelong hematopoiesis after birth, including the quiescent state of adult HSC [23]. Interestingly, osteoclasts, stromal cells essential for hematopoietic niche homeostasis, are EMP-derived cells [24]. In contrast to YS- and AGM-derived macrophages and mast cells, differentiation of the neutrophil lineage, dendritic cells (DC), and monocytes are considered dependent on the fetal BM niche in humans [23, 25] and mice [26]. B cell lineage expansion is a major feature of second trimester fetal BM hematopoiesis [23]. T cell differentiation and maturation on the other hand occur mainly in the fetal thymus and start with the onset of fetal liver hematopoiesis (6–9 PCW) [27].
Tissue-resident immune ontogeny through the lens of fetal macrophage development
The establishment of tissue compartmentalization and lifelong self-sustained immune cell populations originating from embryonic precursors is best described for macrophages in developing mouse tissues [11, 13], which is accompanied by limited but readily increasing human evidence (reviewed in [28]). A nowadays outdated concept hypothesized that tissue-resident macrophages arise from blood monocytes differentiating in the BM, known as the “mononuclear phagocyte system” [29]. Methodological achievements like fate-mapping experiments revealed that certain cell types in certain tissues originate from different developmental pools of precursor cells of early and late embryonic, neonatal, and adult stages. The complex composition of immune cells of different origins is termed “layered immune system,” where certain cell types have distinct origins ranging from entirely or partly fetal- to adult-derived precursors. This concept is for instance apparent in the establishment of tissue-resident macrophages, mast cells, γδ T cells, and ILCs [7, 14, 30–32].
In addition to the primitive and definitive waves of hematopoiesis, another wave of “transient definitive hematopoiesis” from multipotent progenitors (EMPs and lymphoid–myeloid progenitors (LMP)) gives rise to early lymphoid populations and tissue-resident macrophages via a monocytic precursor in mice [33]. Importantly, this wave is still independent of long-term HSC and includes YS EMPs, which seed the fetal liver, where they develop into multiple myeloid lineages including fetal monocytes until mouse embryonic day (E)16.5 [34]. These monocytes seed virtually all embryonic tissues except the brain after mouse midgestation and adapt their transcriptional program to their niche to become specialized tissue-resident macrophages [33]. Tissue-resident macrophages are, to varying levels, self-maintained in postnatal tissues, independently of definitive HSC and replenishment by blood monocytes in steady-state conditions [35, 36]. Prominent examples of these macrophages of strict embryonic origin that are independent of circulating macrophages include brain microglia [37], epidermal Langerhans cells [38], and alveolar macrophages [39]. Other populations can be gradually replaced by adult HSC–derived monocytes, including liver Kupffer cells, splenic, and intestinal macrophages [36, 40, 41].
In an attempt to explain the layered nature of tissue-resident macrophage populations across tissues and ages of an individual, Guilliams and Scott [42] proposed a niche model, hypothesizing that during organogenesis, tissue niches are created (e.g., in the brain) and become available for a limited number of premacrophages from the YS (first primitive wave), which in turn, upon engraftment, receive signals to adopt a tissue-specific phenotype. With continued development, more tissue niches become available for the second transient-definitive wave of embryonic precursors (fetal liver–derived monocytes) until the niche is full. Accordingly, the contribution of definitive HSC-derived monocytes to neonatal tissue composition would be attributable to continued organ maturation after birth. Replacement only occurs when spots in the niche become available. Differences in niche availability for replacement with circulating precursors (i.e., liver, spleen, and intestine available vs brain, skin, and lung being unavailable) might stem from absence or presence of self-renewal-promoting cues, respectively, which are likely to be highly tissue- and/or precursor-specific [42, 43].
During embryogenesis, tissue-resident macrophages contribute to differentiation, maturation, and remodeling of fetal organs, as described in detail for the brain below. Epidermal Langerhans cells are first observed in human fetal skin at 4–5 PCW [44]. In adult skin, they exhibit a specialized phenotype which includes the capacity to repopulate and present antigen, amidst supporting barrier function and repair, and contributing to extracellular matrix remodeling [32, 44–46]. While their developmental function remains incompletely understood, fetal Langerhans cells present a mature phenotype by 16 PCW [47, 48]. Prenatal skin macrophages have been suggested to support angiogenesis and chemotaxis [48]. In the fetal liver, fetal Kupffer cells are considered to adopt similar functions as their adult counterparts, coordinating erythropoiesis and iron recycling [49, 50], responding to bacterial compounds by secreting bona fide tissue-resident macrophage cytokines TNFα, IL-6, and IL-1β in early gestation humans [51, 52], and showing high proliferative capacity, as well as peroxidase activity in mice [53]. The tissue-resident macrophages of embryonic origin of the spleen, red pulp macrophages, are potent iron recyclers and removers of senescent erythrocytes from the neonatal circulation in mice [54, 55], a role they may assume during embryogenesis.
Fetal tissue-resident lymphoid cells
Fetal lymphoid cells partially derive from the wave of transient definitive hematopoiesis prior to definitive hematopoiesis and include NK cells [56], likely lymphoid tissue inducer cells (LTi) [30, 57], as well as marginal zone and B-1 B cells [58], and thymus-derived fetal γδ T cells [32, 59], where, for the latter two, a transient HSC precursor has been described in mice that disappears with adulthood [60]. Similar to myeloid cells, fetal lymphoid cells possess functions that support prenatal development. B-1 cells, considered part of the innate immune system, function in a T cell–independent manner by spontaneously secreting antibodies with limited specificity in peripheral tissues [61, 62], conferring the fetus with unspecific yet immediate immune response capacity. Fetal EMP-derived NK cells are highly potent cytotoxic cells, yet hyporesponsive to HLAneg target cells compared to their adult HSC–derived counterparts [3, 56]. The early developmental origin of human ILC lineages has recently been mapped across fetal tissues during early gestation (8–12 PCW), establishing their varying proportions in and proliferative (i.e., differentiating) states per organ [63]. In a fate-mapping model in mice, prenatal ILC were gradually replaced postnatally, suggesting potential specific roles in their various prenatal tissue sites [30], which will require further investigation. Thymus-derived lineages of fetal T cells derive from fetus-exclusive HSC [64] and appear in the periphery with the start of the second trimester to seed empty niches in lymphoid and mucosal tissues [65–67], reviewed in [68]. In humans, T cells of all lineages are established in utero after gestational week 14 [69–71], possessing innate-like [72], protective [5, 73, 74], and tolerogenic [65, 66, 75] immune response capacity. This is in stark contrast to mice, who only develop T cells after birth [76]. Of note, circulating fetal T cells in the cord blood at term primarily show a naive phenotype and an impaired response towards alloantigens [77], while T cells in the early fetal intestine possess an effector memory phenotype with clonal diversity [5, 66, 74]. This human prenatal T cell landscape adequately exemplifies the compartmentalization of fetal immunity across tissues: systemic, circulating immune cells do not necessarily reflect tissue-resident phenotypes and functions of self-sustained immune cells that contribute to local organogenesis and immunity later in life.
We display current seminal evidence for the human fetal immune landscape and their functional attributes in Fig. 1, and summarize human and mouse studies of fetal immune subsets across tissues in Table 1. Overall, at least in mice, the contribution of definitive hematopoiesis in fetal liver, and later fetal BM, to prenatal tissue-resident immunity has been suggested to be smaller than the contribution from EMP and LMP, which are giving rise to the majority of cell lineages supporting fetal organ development. As proposed by Hoeffel and Ginhoux [33], the stemness of BM-HSC might be preserved until fetal progenitors are fully consumed and have established tissue residency. Furthermore, as proposed by many, including [6], fetal stem and progenitor cells that sense maternal perturbations (infection/inflammation/microbiome/metabolic state) might give rise to progenitors with altered persistence, differentiation potential, and cellular output. Such impact on progenitor populations might amplify into deviations in long-term immune homeostasis and immunity later in life. Consequently, improving fetal and neonatal health will depend on a thorough understanding of prenatal-specific immunity in a spatiotemporal manner.
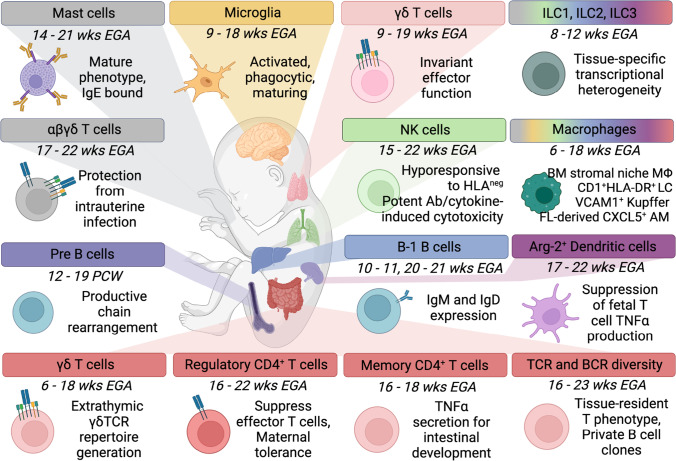
Fig. 1.
Tissue-resident immune populations and functional features in human fetuses. While still incomplete, the current landscape of innate and adaptive immune populations residing in developing tissues during the first and second trimester indicates their role in organogenesis while providing protective/reactive and tolerogenic immunity. Representative seminal evidence is shown. Tissue-resident macrophages adopt specialized phenotypes supporting their niche development, including microglia in the central nervous system [2], Langerhans cells (LC) in skin [44], Kupffer cells in the liver [12], stromal macrophages (MΦ) in the bone marrow [23], and potentially red pulp [55] and alveolar macrophages (AM) [108, 117]. Similarly, innate lymphoid cells (ILC), bona fide tissue-resident cells, are distributed across tissues displaying site-specific phenotypes with as yet incompletely characterized function during fetal life [63]. Lung NK cells possess potent antibody (Ab)- and cytokine-induced cytotoxicity, yet are biased towards tolerating HLAneg cells [3]. In the skin, mature mast cells are sensitized towards allergens via IgE [186], and differentiation of erythroid progenitors might supplement fetal liver erythropoiesis [12]. Innate-like B1 B cells are prominent in the fetal liver and bone marrow, concomitant with naive and memory B-2 B cells [61, 62], while B cell clonality in the gut is primarily private, and not shared between individuals [82]. B cell lineage expansion occurs in the second trimester bone marrow [23]. Spleen dendritic cells (DC) have antigen-presentation capacity, supporting tolerogenic T cell responses [110]. T cells have an innate-like, fast-response phenotype (invariant γδ T cells) in the thymus [59, 229], skin [73], and intestine [59], however, are strongly biased towards mediating (maternal) tolerance [66, 71, 75], yet show TCR diversity [67, 82] and effector memory [5, 67, 82] in the mucosal surface of the gut. Cord blood CD71+ erythrocytes (not shown) are perinatal immunosuppressors, potentially derived from fetal liver erythroid precursors [12, 244, 245]. Created with BioRender.com
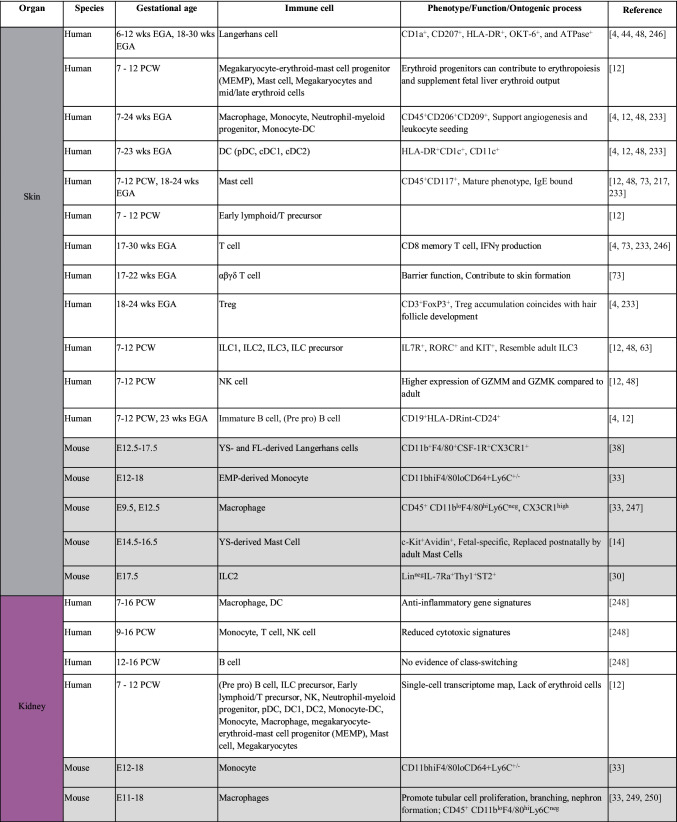
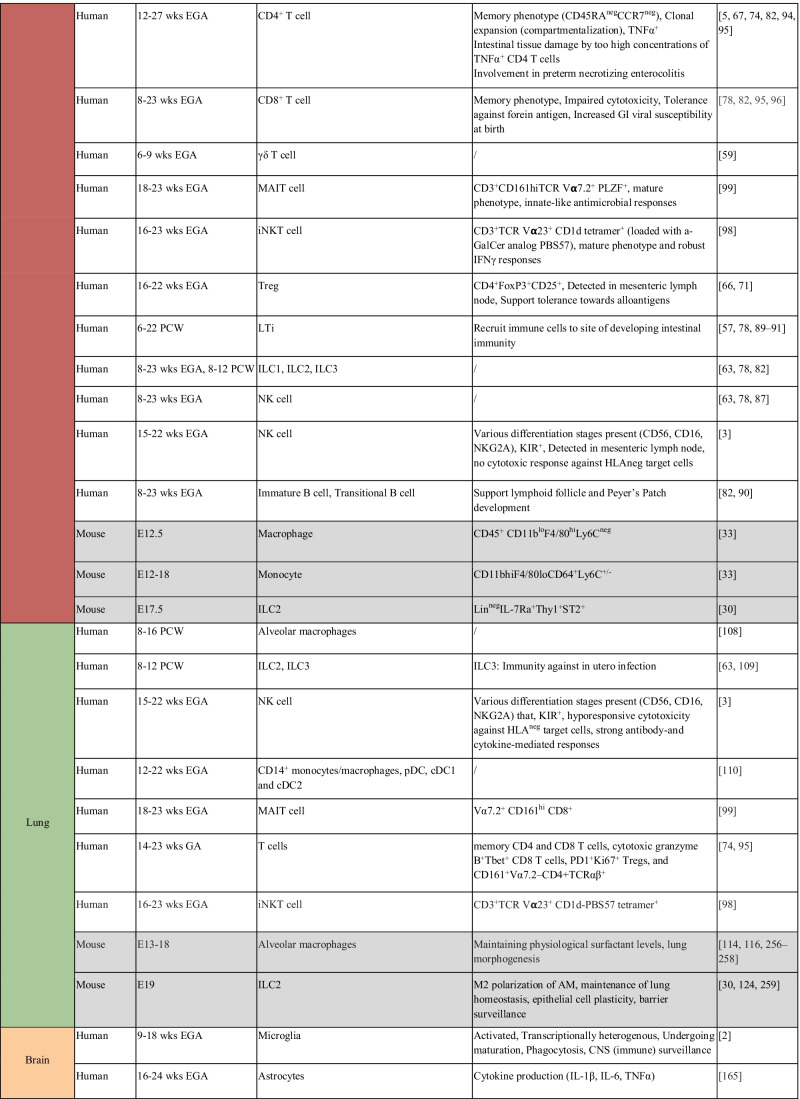
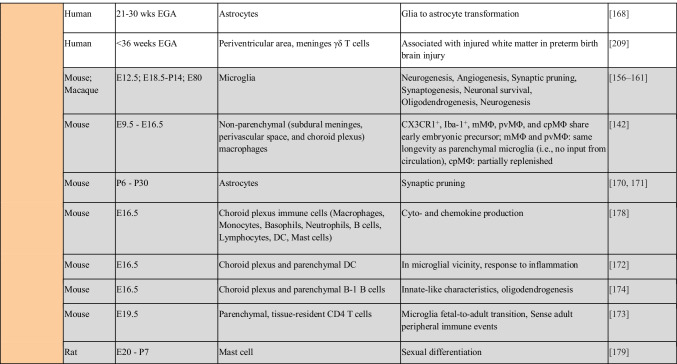
Table 1.
Immune populations resident in human and mouse fetal tissues. Color-coding of table mirrors tissue highlighted in Fig. 1. Information regarding non-human models is indicated in gray. E12.5, embryonic day 12.5; YS, yolk sac; FL, fetal liver; EMP, erythro-myeloid progenitor; ILC, innate lymphoid cell; DC, dendritic cell; pDC, plasmacytoid DC; cDC, conventional DC; Treg, regulatory T cell; DN, double negative; DP, double positive; EGA, estimated gestational age; GI, gastrointestinal tract; PCW, postconception week; LTi, lymphoid tissue inducer; MAIT cell, mucosal-associated invariant T cell; mMΦ, pvMΦ, cpMΦ, subdural meninges, perivascular space, and choroid plexus macrophages; P, postnatal day; iNKT cell, invariant natural killer T cell; KIR, killer immunoglobulin-like receptor; wks, weeks
Development and function of resident immunity in the fetus
Mucosal immune populations in the fetal gut
As the largest barrier organ in the human body, the intestine has to coordinate nutritional demands and immune symbiosis with the gut microbiome [78]. Especially after birth, the mucosal intestine gets bombarded by environmental, nutritional, and microbial exposure [79]. As a result, the intestine needs to balance innocuous responses to these exposures, while at the same time provide protection against potential pathogenic exposure. Unfortunately, differences in intestinal development between humans and animal models — mice are born with an immature gastrointestinal mucosa [79] — highlight the hurdles and difficulties of studying human intestinal immune development. However, recent technological advances have made it possible to study the development of the mucosal intestine in precious human fetal tissue.
Several studies have emphasized that the human fetal intestine contains a distinct tissue-specific immune composition and signature [80] that consists of antigen presenting cells (APC), ILC subsets, T cells, γδ T cells, and B cells which can already be observed in human fetal intestine at 6 to 23 weeks of gestation [59, 63, 67, 78, 80–82]. A recent scRNAseq study performed in intestinal tissue from 17 human embryos, ranging from 8 to 22 weeks post conception, suggest that intestinal immune cells are rare prior to the first trimester [78]. However, an enrichment of myeloid cells could be observed before 10 PCW, whereas an influx of T cells, NK cells, ILC1, and ILC3 were observed at 12 PCW [78], coinciding with the development of gut-associated lymphatic tissue (GALT) [79, 83–85]. The basic intestinal immune landscape — proportions of major innate and adaptive immune cells — is established prior to the second trimester and is stable into infancy [82]. However, diversity within the major immune cell populations can be observed, with an enrichment of CD11c+ APC, ILC, and CD4+ T cells, and reduced plasma cells in the fetus compared to infant [82]. Interestingly, in the adult, maintenance of intestinal mucosal immunity occurs both through migration of circulating BM-derived cells and by differentiation from tissue-resident progenitor cells that were seeded during fetal development [86]. For instance, macrophages are replenished by circulating monocytes [41], while ILC subsets and their progenitors reside in the tissue itself [63, 81, 87]. Also T cell progenitors reside in the intestine itself, as intestinal T cell differentiation is independent of the thymus [59, 88]. In humans, GALT, including lamina propria, mesenteric lymph nodes (mLN), and Peyer’s patches (PP), begins to develop 11–12 PCW, and at gestational week 19 the fetal intestines reach full structural maturity [79, 83–85, 89].
Several non-immune cells such as mesenchymal and epithelial cells are important for development of intestinal immunity [57, 78, 89–91]. It has been shown that interactions between mesenchymal lymphoid tissue organizing (mLTo), endothelial LTo (eLTo), and LTi cells — which belong to the ILC family — are critical for recruiting immune cells to the site of the developing intestinal immunity [57, 78, 89–91]. In addition, epithelial cells in the fetal intestine will produce chemerin to attract macrophages from the circulation into the fetal gut mucosa [92], while after birth IL-8 and TGFβ secreted by epithelial cells and mast cells will attract macrophages to the intestinal mucosa [93].
The general hypothesis that the fetal immune system is naive and will only mature after birth has been challenged by the observation that a relatively mature adaptive immune system is present in the fetal gut [82]. For instance, clonally expanded CD4+ T cells with a memory phenotype (Tm) [5, 67, 74, 82, 88, 94, 95] show no overlap with regards to clonality in blood, and are present in the fetal intestine at 13–23 weeks of gestation [5, 67, 88, 94], highlighting compartmentalization of the fetal immune system. T cell receptor repertoire diversification and clonal distribution of intestinal T cells starts in utero and continues after birth [82]. These fetal CD4+ Tm cells can be found in colocalization with APC in the fetal intestine and are able to produce pro-inflammatory cytokines upon stimulation [5, 67, 74]. In particular, intestinal TNFα+ CD4+ Tm cells are able to support intestinal stem cell growth and epithelial development [5]. Importantly, a too high concentration of TNFα-producing CD4+ Tm cells mediates intestinal tissue damage, which is in line with the observation of increased frequencies of TNFα+ CD4+ Tm cells in the intestine of necrotizing enterocolitis (NEC)–affected preterm infants [5]. Overall, these data showed that TNFα-producing CD4+ Tm cells are involved in tissue generation in the fetus, while preterm birth-induced activation can induce intestinal inflammation and damage in premature infants. CD8+ T cells with a memory phenotype [95, 96] can also be detected in the fetal intestine, with proportions increasing after birth. However, fetal and infant intestinal CD8+ T cells showed reduced functional capacity compared to adult intestinal CD8+ T cells [96], which is in contrast with results observed in mice [97]. This impaired cytotoxic CD8+ T cell immunity suggests a tolerance support towards foreign antigens after birth, while at the same time it might contribute to the increased gastrointestinal viral susceptibility observed during early life. The expression of CD69 and CD103 on both CD4 and CD8 Tm cells suggests that these intestinal T cells are of the tissue-resident memory (TRM) phenotype [82]. Also γδ T cells [59], invariant NK T cells [98], and mucosal-associated invariant T (MAIT) cells with a mature phenotype and innate-like antimicrobial responses are present in human fetal intestine [99]. The presence of these TRM T cells suggests in utero exposure to foreign antigens, potentially mediated through swallowing of amniotic fluid by the fetus as early as 11 weeks of gestation (EGA) [100, 101]. Mishra et al. detected a low but consistent microbial presence in human fetal organs, including the gut, and with live fetal-isolated microbial strains capable of inducing memory T cell activation and proliferation in vitro [95]. Even though this study suggests that fetal microbiota are capable of educating fetal intestinal T cells, the presence of microbiota in utero is still very controversial [102–104], predominantly due to the lack of appropriate controls and methodological limitations. Nevertheless, it remains imperative to elucidate which agents are responsible for in utero maturation of intestinal T cells.
B cells, on the other hand, have a relatively immature phenotype in the fetal gut, and maturation will occur after birth [82, 90]. In the fetal intestine, an enrichment for transitional B cells can be observed, a subset that has recently migrated from the BM and/or liver, while an enrichment of IgM+ plasma cells can be observed in the intestine of infants [82]. The in utero function of B cells is likely to support the development of lymphoid follicles and PP, as observed in mice [105]. After birth, the expansion of plasma cells will likely shape the selection of a healthy and beneficial intestinal microbiome.
Mucosal immune populations in the fetal lung
Lung-resident immune cells are key to mount immunity towards airborne pathogens to which the airways and interstitium are permanently exposed [106]. Mature DC and alveolar macrophages (AM) are among the first cells to encounter invading microbes [106]. Although the fetus is not exposed to airborne pathogens and the lung continues to mature postnatally into the first years of life [107], immune cells and precursors already seed the fetal lung, establishing immunity to upcoming lifelong challenges. A distinct immune cell repertoire can be detected in the human fetal lung as early as 8 weeks post conception [3, 63, 74, 95, 98, 99, 108–110], coinciding with the start of the pseudoglandular stage of lung development (i.e., differentiation of epithelial cells, formation of conduction airway and terminal bronchioles, formation of pulmonary blood vessels) [107].
AM are the most abundant immune cell type in the adult lung, maintaining lung homeostasis through clearance of debris, invading pathogens, and pulmonary surfactant (reviewed in [111–113]). Macrophages scattered in the fetal lung have been detected around 13–17 PCW in humans [108, 110], and at E13.5 in mice [114]. Currently, little is known about the origin and phenotype of human fetal AM. In mice, however, lung development occurs in parallel with seeding of fetal liver-monocyte derived AM, a self-replenishing population whose maturation is dependent on GM-CSF [39, 115] and maintained until adulthood [36]. The role of fetal liver–derived monocytes in the establishment of AM in the murine fetus was confirmed in a fate-mapping approach [33, 34, 116]. Different reservoirs of fetal monocytes, i.e., BM-derived monocytes, YS-derived macrophages, and fetal-liver monocytes, were tested for their ability to serve as AM precursors. Fetal liver–derived monocytes showed superior GM-CSF responsiveness based on their proliferation-associated gene expression yielding in higher proliferative capacity in vitro [116], showed upregulation of bona fide macrophage differentiation markers such as CD64 and MerTK upon seeding of future resident tissue in vivo, and outcompeted YS macrophages and BM monocytes in colonizing an empty AM niche [33]. In addition, using humanized mouse models, it has been confirmed that human fetal-liver AM precursors are able to seed the lung, giving rise to AM [117].
Next to macrophages, also DC and NK cells will be one of the first immune cells to encounter invading pathogens in the newborn and adult lung. Although lung-resident DC are well characterized in the adult lung, information on DC in the developing fetal lung is limited. However, gene array and flow cytometry analyses have revealed insights into the presence of human fetal lung DC at gestational week 12–22 [110], including plasmacytoid DC (pDC), conventional (c)DC1, and cDC2, showing tissue-specific phenotypes. Data from human fetal lungs obtained at gestational week 15–22 showed that NK cells were present in the mesenchyme of the developing lung tissue [3]. NK cell differentiation and killer immunoglobulin-like receptors (KIR) acquisition starts early, likely before 15 weeks of gestation in the developing fetal lung, with fetal lung NK cells being the most differentiated in comparison to fetal liver, BM, lymph node, and spleen NK cells [3]. This suggests that NK cell differentiation occurs within the lung itself. While fetal lung NK cells are less differentiated compared to circulating adult NK cells, their high expression of multiple KIR and intracellular expression of perforin and granzyme B suggests that they may be functional [3]. Indeed, in agreement with fetal liver NK cells [118], fetal lung NK cells show degranulation and cytotoxic activity against HLAneg target cells, albeit to a lower extent than their adult peripheral counterparts [3]. In contrast, fetal lung NK cells were highly responsive to cytokine stimulation and antibody-dependent killing of target cells [3]. Interestingly, upon IL-12 stimulation, the percentage of fetal lung NK cells that express IFNγ are higher than adult peripheral NK cells [3]. In conclusion, although fetal lung NK cells are hyporesponsive to cells lacking HLA class I, they possess a strong cytokine- and antibody-mediated immune response capacity, suggesting a balance between fetal-maternal tolerance while still maintaining the ability to respond to infection in utero.
Characterizing ILC populations across human fetal tissues revealed a transcriptomic map of diverse subpopulations with site-specific expression profiles in 8–12 PCW fetuses [63]. ILC1, ILC2, and ILC3 subsets can be found in the fetal lung, with ILC3 being the predominant ILC subset [63]. During murine fetal growth, ILC are involved in the development of secondary lymphoid organs (spleen, lymph nodes, and PP) [119–121], while in non-lymphoid tissues, such as the intestine and lung, mature ILC are considered to establish immunity during colonization and after birth (reviewed in [122, 123]). Increasing frequencies of a CD103-expressing subset of ILC3, indicating intraepithelial origin, have been identified in the human fetal lung with increasing gestational age (8–20 weeks EGA) [109]. At 20 weeks EGA, these CD103+ ILC3 will make up 15% of ILC3 in the fetal lung [109]. These cells can also be detected in the amniotic fluid at 8–20 weeks EGA, likely through migration from the fetal lung or fetal intestine, where they are believed to mount immunity against in utero infections [109]. Results from lineage tracing and parabiosis experiments in mice have shown that mature ILC2, identified by markers such as Linneg IL7Ra+ Thy1+ ST2+, are present in the lung at E17.5 [30]. An extensive proliferation of ILC2 occurs in the murine lung at the time of birth, which is linked to the first breath [124]. This first breath leads to a mechanical stimulus that induces IL-33 secretion by type 2 alveolar epithelial cells, which is able to activate a variety of cells in the lung such as regulatory T cells (Treg), DC, mast cells, NK cells, AM, and ILC2 [124]. Due to the activation by IL-33, ILC2 of newborn mice start to secrete IL-13, which in turn drives AM polarization towards an M2 phenotype [124]. Two months after birth, only 5–10% of tissue-resident ILC2 are embryonically derived, suggesting a gradual exchange of the tissue-resident pool by circulating ILC [30].
Recent reports show that adaptive immune cells present in the second trimester human fetal lung (13–23 weeks EGA) include memory CD4 and CD8 T cells, cytotoxic granzyme B+ Tbet+ CD8 T cells, PD1+ Ki67+ Tregs, and CD161+ Vα7.2–CD4+ TCR-αβ + T cells [74, 95]. Furthermore, the second trimester human lung harbors a population of innate-like Vα7.2+ CD161hi CD8+ MAIT cells, with IFNγ and IL-22-producing capacity [99], and invariant NKT cells (CD1d-PBS57 tetramer+ CD3+ TCRVα24+) [98]. Beyond their detection and phenotype-based functional implications, the role of T cells for prenatal lung development and immunity remains to be investigated.
Although not immune cells per se, clinical studies in humans suggest that mesenchymal stromal cells (MSC) possess immune-modulatory properties making them a potential target for cell therapy in idiopathic pulmonary fibrosis (IPF) [125] and acute respiratory distress syndrome (ARDS) [126]. Fetal lung-resident MSCs show lung-specific properties that are distinct from BM-derived and adult lung-resident MSC, and play a role during lung development [127]. They produce higher amounts of macrophage migration inhibitory factor (MIF) when compared to adult lung-resident MSC, a chemokine which hinders random macrophage migration and supports lung development by promoting growth of pulmonary arterial smooth muscle cells [128]. Moreover, MIF is expressed tenfold higher in newborns compared to children and adults, underpinning its involvement in lung development [128, 129].
Parenchymal and border-associated immune populations in the fetal central nervous system
Neurodevelopment of the human central nervous system (CNS) follows spatially and temporally highly orchestrated patterns (reviewed in [130]). Knowledge about the development of tissue-resident immunity in the brain during the fetal period remains incomplete. Even in the adult CNS, steady-state immune surveillance of brain surfaces was only recently characterized more comprehensively [131–134]. In the parenchyma, homeostatic tissue residency is dominated by microglia, the primary brain macrophage population, while central and systemic inflammation generates a small population of resident T cells [135–137]. Immune and non-immune roles of microglia in brain development have been extensively described in physiological and pathological conditions ([138], reviewed in [139, 140]).
Microglia colonize the brain early in development. Their origin has been revealed in studies in rodents, showing that microglia are derived from primitive and YS EMP–derived precursors who establish themselves in the neuroectoderm by E9.5 [37, 141–143]. Rodent infiltrated microglia locate around subcortical regions (hippocampus) and corpus callosum on E13.5, then expand and migrate into distant brain regions for final residency, proliferating beyond birth following a precisely timed transcriptional program [144]. From rodent studies, it is known that their numbers contract in postnatal week 3 and subsequently stabilize to adult levels after week 4 [145, 146]. Microglial colonization coincides with rodent brain maturation, and so does their morphology. Developmental, immature microglia are amoeboid (large and round) in shape, resembling activated adult microglia, and are dramatically different from the thin, ramified morphology of adult microglia at steady state, a morphology they assume in parallel with increasing maturation of individual brain regions after birth [147]. In humans, microglia are spotted in close mesenchymal vicinity to neural tissue by 4.5 weeks gestation, and in the neural tissue 1 week later, responding to chemokine signals [148–152]. Recently, human fetal microglia have been transcriptionally characterized on the single-cell level ranging from 9 to 18 weeks EGA, displaying tremendous heterogeneity, and undergoing dynamic processes of maturation to acquire a homeostatic profile, with overlapping and distinct features compared to adult microglia [2].
Microglia are macrophages and, as such, actively engaged in phagocytosis of cellular debris and opsonized synapses, apoptotic clearance, and induction of apoptosis [153–155]. In addition, microglia support a variety of non-immune developmental processes of the macaque and rodent fetal brain including neurogenesis [156] and angiogenesis [157]. In mouse late gestation and early neonatal periods, they contribute to complement-mediated synaptic pruning [158], synaptogenesis [159], survival of neurons [160], oligodendrogenesis, and myelination [161]. This variety of microglia functions depends on time and localization. As one type among tissue-resident macrophages derived from the first primitive and YS EMP–derived premacrophages, microglia are distinct from their counterparts residing in peritoneum, liver, or lung, due to the specific microenvironment in the CNS that primes their functional capacity [34, 43, 162]. Importantly, coinciding with maturation of the blood–brain-barrier (BBB) on E13 in mice [163], the brain niche is considered inaccessible for further precursor seeding after E14.5, thus rendering the microglial population derived from fetal origin, and requiring in situ repopulation capacity for the remainder of the individual’s lifespan [42]. In human embryos, the BBB is considered mature around gestational week 8 [163]. Investigating developmental spatiotemporal patterning of human microglia and their progenitors is crucial in understanding both developmental and mature microglia diversity.
The developing brain parenchymal niche is furthermore colonized by astrocytes, a neural stem cell-derived, yet highly immunocompetent cell type. Their interaction with microglia and other infiltrating immune cell subsets makes them an important component of brain immunity, as they respond to and secrete inflammatory mediators (reviewed in [164]). For example, during the human second trimester, astrocytes produce TNFα and IL-6 upon IL-1β stimulation, a cytokine produced by LPS-responsive fetal microglia [165]. This promotes synaptic transmission and affects synaptic scaling [166]. Human astrocytes start transforming from glial cells after 12 weeks EGA [167–169]. Similar to microglia, astrocytes have phagocytic activity and engage in synaptic pruning [170], while simultaneously promoting microglial pruning during postnatal development [171].
Conventional immune cell subsets are found at very low frequencies in the unchallenged developing brain parenchyma [172–174]. In the adult brain in steady state, the majority of tissue-resident immune cells are non-parenchymal immune subsets residing in the organ border such as the dura mater, subdural meninges, and choroid plexus, and on the abluminal side of the BBB, to surveille and influence mature CNS function [131–133, 138, 175]. These brain-border-associated immune subsets in the choroid plexus include both adaptive and innate immune cells and show a phenotype distinct from microglia [138] and circulating immune cells, indicating their selective enrichment [134]. The choroid plexus is an epithelial bilayer that forms the blood-cerebrospinal fluid (CSF) barrier (BCSFB), that, in addition to immune cells, hosts mesenchymal, and neuronal cells. Its role for establishment of the fetal brain immune landscape across time and space remains scarcely described, while its involvement in mouse brain immune surveillance and embryonic neuroinflammation has been documented [176, 177].
A number of recent studies are starting to build a narrative of parenchymal and brain border-associated immune cell presence establishing in utero. The fetal immune composition in the choroid plexus on E16.5 in mice resembles both the adult and aging brain, with regard to gene expression patterns in B and T cells, macrophages, monocytes, basophils, DC, neutrophils, and mast cells, while unique embryonic features were identified as well [178]. For example, border-associated macrophages in the embryonic choroid plexus express CD206 and are MHC class IIlow, compared to their adult CD206low and MHC class IIhigh (i.e., CD74) counterparts. They are also enriched for the expression of a set of markers (e.g., Lyve1) assigned to adult subdural-specific macrophages [162, 178]. Perivascular, meningeal, and choroid plexus non-parenchymal macrophages, previously assumed to be short-lived and continuously replaced by adult BM-derived circulating cells, were determined to share their embryonic origin with YS-derived parenchymal microglia (wave of transient definitive hematopoiesis) [142], indicating that these precursors are present during fetal brain development. Bulloch et al. describe another myeloid subset, CD11c+ DC, in the choroid plexus, but also in the murine brain parenchyma on E16.5, co-localizing with microglia. In adult mice, these same cells responded to injury with microglia-like morphology [172]. At the same fetal age, Tanabe and Yamashita isolated T and B cells from the mouse brain, finding abundant innate-like B-1 cells expressing high levels of IgM [174]. These cells localize in the choroid plexus and meninges and, rarely, in the cortical parenchyma on postnatal day 1. They followed CXCR5-dependent recruitment in response to CXCL13 secreted from the choroid plexus, and promoted the proliferation of oligodendrocyte precursor cells (OPC) in vitro. This B-1 population was highly transient, reaching its peak frequency 1 day after birth, and disappearing by postnatal day 10. A small but functionally significant CD4+ T cell population with a brain-resident phenotype is found in the adult human and mouse brain at steady state, with a subset localizing in non-vascular, non-meningeal, parenchymal spaces [173]. Of note, these brain-resident CD4+ T cells, although rare in general, were particularly frequent in the fetal mouse brain right before delivery, when they were supporting the early-life transition of microglia from a fetal to mature transcriptional program, ultimately enabling proper synaptic pruning [173]. Anecdotal evidence for the involvement of non-microglial immune cells in organogenesis describes the estradiol-dependent involvement of mast cells in the development of sexual differentiation in the preoptic area in the rat [179].
Evidence for immune memory in the fetus
To complete the porous picture on local fetal immunity in humans with the generally better described system-level perspective, we will highlight evidence for fetal priming of immune function in humans and rodents, based on the mechanisms of innate trained immunity and adaptive antigen-specific memory.
The developing, circulating fetal immune system senses the maternal circulating environment at the fetal-maternal interface. The fetus is exposed to maternal antigen [66], maternal-derived pathogen-associated molecular patterns (PAMPs), DNA and other products from the mother’s commensal microbiota [180–183], maternal antibodies (including passive immunity) [184–186], cytokines [180], and to vertical transfer of pathogens or their products, i.e., transmission of infection [187, 188], as well as vertical transfer of intact maternal cells (220). These maternal microchimeric cells seed across fetal organs to become part of the tissue-resident population with as yet incompletely described consequences for the fetus and neonate [189, 190]. These exposures may be categorized as inherent maternal support and education, and therefore be considered beneficial for fetal immune development. However, the fetus sensing maternal (immune) perturbations, as described in the concept of the developmental origins of health and disease, or “Barker hypothesis,” may have lifelong detrimental consequences for offspring’s immune health (reviewed in [6, 7]), as highlighted in Box 1 for prenatal immune activation of tissue-resident immune populations.
Box 1.
Prenatal immune activation of tissue-resident populations and developmental disorders later in life
| Evidence for postnatal consequences resulting from prenatal disturbances of resident immune populations are emerging for gut and lung, and have been extensively described for the brain. In the gut, transient prenatal maternal infection at E10.5 caused an increase in intestinal Th17 cells in the adult offspring that, while enhancing protection against gut infection, also increased susceptibility to colitis [180]. In addition, fetal exposure to chorioamnionitis has been associated with dysregulation of fetal gut immunity, intestinal inflammation, and the development of necrotizing enterocolitis weeks after birth [191, 192]. While the exposure of the lung to infections during the neonatal and adult phase is known to lead to chronic lung diseases, less is known about the consequences of inflammatory exposure on fetal lung immune development. In utero, the fetal lung is constantly exposed to the amniotic fluid and pregnancy complications like chorioamnionitis can lead to an inflammatory milieu within the amniotic fluid, resulting in increased leukocyte infiltration, surfactant production, decreased alveolarization (reviewed in [193, 194]), maturation of monocytes to alveolar macrophages, and both the induction as well as the paralysis of inflammatory responses in the fetal lung [195]. In the brain, an association exists between immune activation during embryogenesis, and neurodevelopmental disorders later in life, such as autism spectrum disorder, schizophrenia, and obsessive compulsive disorder [196–198]. Maternal stress/anxiety, nutrition, or infection (e.g., maternal immune activation (MIA)) can program the developing neural structures with long-term sequelae for behavior, cognition, and brain immunity [199–202]. For instance, early-life viral infection is considered to be associated with the adult onset of schizophrenia [197, 203, 204]. Aberrant microglia priming during development as a mechanistic link between immune activation and developmental disorders has been reviewed in [139, 196]. |
| Briefly, mediators of prenatal immune activation and impaired neurodevelopment could be the vertical transmission of an infection, maternal antibodies that react with fetal neural tissue [205], microbiome alterations [206], or the transfer of an inflammatory milieu of maternal cytokines, PAMPs, or viral particles that in turn activate the fetal immune system itself [6, 207]. While glial-derived cytokines are critically contributing to normal brain development, elevated levels of systemic and central IL-1β, IL-6, and TNFα have been shown in experimental models to affect neural development and impair learning and memory, as well as behavior in rodents [208–211], via altered microglial and astrocyte functions, including microglial activation [139, 165, 212]. Increased permeability of the BBB and BCSFB in response to inflammation allows for infiltration of circulating and resident immune cells, and other immune mediators such as cytokines and antibodies into the brain parenchyma [177, 213–215]. |
| In the adult mouse brain, microglia and border-associated macrophage populations show a highly reactive state in response to a neuroinflammatory microenvironment [138], which is supported by parenchymal infiltration of a non-resident BM-derived monocyte population to the site of brain injury [216]. In addition, CNS tissue-resident T cells establish memory after local and systemic infection [135, 137], and can sense microbiome alterations and antibiotic treatment in the periphery [173]. |
| In the perinatal human brain, neuroinflammation and brain injury are common in infants born prematurely (reviewed in [217, 218]). Astrocytes and microglia secrete cyto- and chemokines causing leukocyte infiltration [139]. In humans and mice, accumulated γδ T cells have been found to fuel fetal brain inflammation via IFNγ and non-canonical (i.e., not IL-17) signals [219–221]. Peripheral microbial composition (i.e., expanded proportion of Klebsiella in the gut) correlates with inflammation and is predictive of the severity of brain injury in extremely preterm infants [220], emphasizing a gut-immune-brain axis with likely prenatal origin that links neuro- with systemic development. |
The fetal innate immune system might be trained by these transplacental exposures, leading to persistent transcriptional and epigenetic changes in innate cells and their capacity to respond to secondary challenges unrelated to the primary trigger/pathogen, a concept termed “trained immunity” in adult immune responses [222]. For instance, in utero exposure to hepatitis B virus (HBV) leads to a more mature monocyte phenotype and function in cord blood of uninfected neonates, most likely mediated by changes in cytokine environment, and affects T cell polarization, indicative of the concomitant effect on the adaptive arm [223]. Similar observations of altered immune capacity were made for placental malaria [224] and human immunodeficiency virus (HIV) [225]. In mice, the BM-lung immune axis was trained following maternal microbial exposure in utero, leading to protection from inflammatory airway disease via enhanced survival and proliferation of the lung DC population which was programmed in its BM-resident precursor [226]. Whether pathogen-nonspecific immunity in uninfected fetuses and neonates resulting from maternal inflammation/infection exists and is inherently beneficial for offspring’s health warrants further research [227, 228].
Adaptive fetal immune memory in the periphery is observed after gestational week 14, following thymic emigration and seeding of peripheral organs which equips the human fetus with the complete repertoire of T cell subsets [69–71]. The fetal environment prioritizes unique phenotypes of T cells, including a strong bias towards immune tolerance and the preferential differentiation of naive T cells into Tregs [65]. Thymic [229] and circulating [72] γδ T cells with fetus-specific phenotypes serve fast-acting functions, in addition to innate-like features of CD4+ and CD8+ T cells [230, 231], which, in mouse CD8+ T cells, are retained until adulthood [97]. Despite a relative sparsity in foreign antigen load, the fetal immune system recognizes transferred non-inherited maternal antigens by inducing alloantigen-specific Tregs [66, 232] and shows the potential to establish resident CD4 and CD8 memory (with unknown specificity) in the gut and skin in utero [5, 74, 96, 233]. Fetal CD8+ T cell immunity is functional in its antigen recognition, even if less potent (i.e., decreased cytotoxicity) compared to adult cells [231]. Pathogen-specific CD8+ T cell responses are detectable in cord blood, including to cytomegalovirus (CMV) [234], HIV [235], malaria [236], and HBV [237]. Even in the absence of the pathogen itself, the fetal T cell compartment senses foreign antigens, as maternal influenza vaccination is associated with potent antigen-specific T cells in cord blood [238].
While the mechanisms of priming beneficial vs. detrimental trained and antigen-specific memory remain to be investigated, targeted maternal and neonatal immunization strategies to support the development of fetal adaptive and innate immunity could be of immediate (postnatal) and lifelong benefit (reviewed in [228]).
Conclusion: opportunities for pathophysiological discoveries and therapeutic windows
Our knowledge on the capacity of the human fetus to mount tissue-resident immune responses remains incomplete and restricted to samples from early pregnancy. More evidence of innate and adaptive perinatal immunity exists on a systemic level (often cord blood studies), which can only be a snapshot of a spatially complex system and requires complementing data from animal models to complete the picture of organ-specific prenatal tissue residency. Differences between tissue and systemic immunity in the neonate do not allow us to understand (patho)biology within tissues using circulating cells in cord blood; yet, its accessibility makes it a useful source for biomarker discovery.
The dynamic perinatal period is susceptible to external influence. Deviations in programming of fetal immune precursors can have lifelong consequences for the health of the entire organism. This presents opportunities for interventions to support healthy developmental trajectories. Recent technical advances allow the in-depth, high-throughput assessment of a much smaller sample volume to generate high-dimensional maps of spatiotemporal dynamics of organ development in humans. Furthermore, to spur our understanding of human development, human gestational samples, complemented by stem cell-derived organoid models [239–243], instead of rodent, sheep, or non-human primate models with limited overlap in their gestational physiology, are needed to reveal functional overlap and separation of systemic vs. compartmentalized tissue-resident immunity, and identify windows of susceptibility and therapeutic opportunity.
Funding
This work was supported by the Stanford Maternal and Child Health Research Institute—Postdoctoral Funding (DF), the German Research Foundation (FOR 5068; P8, AR232/29-1) (CU), the March of Dimes Prematurity Research Center at Stanford University (#22FY19343); the Bill & Melinda Gates Foundation (OPP1189911); the Stanford Center for Human Systems Immunology; the Stanford Maternal and Child Health Research Institute; and the National Institute of Health (NIH P01HD106414) (BG), and NIH/NICHD (K99HD105016) (IS).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
PCW describes the fetal age since fertilization, which occurs about 2 weeks later than the start of the last menstrual cycle, which, in turn, is used to estimate gestational age (EGA) in weeks. Throughout this review, we report PCW and EGA verbatim from the source reports to avoid introducing inaccuracies.
This article is a contribution to the special issue on: Heterogeneity of tissue-resident immunity across organs and in health and disease - Guest Editors: Federica Sallusto & Petra Arck
The original online version of this article was revised: The article contained an incomplete author list. The name of the author, Brice Gaudillière, was missing. The correct list of authors is shown here: Dorien Feyaerts, Christopher Urbschat, Brice Gaudillière, Ina A. Stelzer. Brice Gaudillière has the following affiliations:
1Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Palo Alto, CA, USA
3Department of Pediatrics, Stanford University School of Medicine, Palo Alto, CA, USA
Further the incomplete funding was corrected
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/12/2022
A Correction to this paper has been published: 10.1007/s00281-022-00954-4
References
- 1.Park J-E, Jardine L, Gottgens B, Teichmann SA, Haniffa M. Prenatal development of human immunity. Science. 2020;368(6491):600–603. doi: 10.1126/science.aaz9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kracht L, Borggrewe M, Eskandar S, Brouwer N, de Sousa C, Lopes SM, et al. Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science. 2020;369(6503):530–537. doi: 10.1126/science.aba5906. [DOI] [PubMed] [Google Scholar]
- 3.Ivarsson MA, Loh L, Marquardt N, Kekäläinen E, Berglin L, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123(9):3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhariwala MO, Karthikeyan D, Vasquez KS, Farhat S, Weckel A, et al. Developing human skin contains lymphocytes demonstrating a memory signature. Cell Rep Med. 2020;1(8):100132. doi: 10.1016/j.xcrm.2020.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreurs RRCE, Baumdick ME, Sagebiel AF, Kaufmann M, Mokry M, et al. Human fetal TNF-α-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity. 2019;50(2):462–476.e8. doi: 10.1016/j.immuni.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Apostol AC, Jensen KDC, Beaudin AE. Training the fetal immune system through maternal inflammation-a layered hygiene hypothesis. Front Immunol. 2020;11:123. doi: 10.3389/fimmu.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mass E, Gentek R. Fetal-derived immune cells at the roots of lifelong pathophysiology. Front Cell Dev Biol. 2021;9:648313. doi: 10.3389/fcell.2021.648313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol. 2019;37:521–546. doi: 10.1146/annurev-immunol-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou C, Li MO. Tissue-resident lymphocytes across innate and adaptive lineages. Front Immunol. 2018;9:2104. doi: 10.3389/fimmu.2018.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain Z, Reza AHMM, Qasem WA, Friel JK, Omri A (2022) Development of the immune system in the human embryo. Pediatr Res. 10.1038/s41390-022-01940-0 [DOI] [PubMed]
- 11.Hoeffel G, Ginhoux F. Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 2018;330:5–15. doi: 10.1016/j.cellimm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Popescu D-M, Botting RA, Stephenson E, Green K, Webb S, et al. Decoding human fetal liver haematopoiesis. Nature. 2019;574(7778):365–371. doi: 10.1038/s41586-019-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353(6304):aaf4238. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, et al. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 2018;48(6):1160–1171.e5. doi: 10.1016/j.immuni.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Julien E, El Omar R, Tavian M. Origin of the hematopoietic system in the human embryo. FEBS Lett. 2016;590(22):3987–4001. doi: 10.1002/1873-3468.12389. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Wang T, Gu J, Huang K, Zhang T, et al. Characterization and generation of human definitive multipotent hematopoietic stem/progenitor cells. Cell Discov. 2020;6(1):89. doi: 10.1038/s41421-020-00213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Y, He J, Bai Z, Li Z, Gong Y, et al. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res. 2019;29(11):881–894. doi: 10.1038/s41422-019-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gekas C, Rhodes KE, Van Handel B, Chhabra A, Ueno M, Mikkola HKA. Hematopoietic stem cell development in the placenta. Int J Dev Biol. 2010;54(6–7):1089–1098. doi: 10.1387/ijdb.103070cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian Z, Gong Y, Huang T, Lee CZW, Bian L, et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582(7813):571–576. doi: 10.1038/s41586-020-2316-7. [DOI] [PubMed] [Google Scholar]
- 20.Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95(7):2284–2288. doi: 10.1182/blood.V95.7.2284. [DOI] [PubMed] [Google Scholar]
- 21.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HKA. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14(5):493–504. doi: 10.1089/scd.2005.14.493. [DOI] [PubMed] [Google Scholar]
- 23.Jardine L, Webb S, Goh I, Quiroga Londoño M, Reynolds G, et al. Blood and immune development in human fetal bone marrow and Down syndrome. Nature. 2021;598(7880):327–331. doi: 10.1038/s41586-021-03929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slayton WB, Li Y, Calhoun DA, Juul SE, Iturraspe J, et al. The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum Dev. 1998;53(2):129–144. doi: 10.1016/S0378-3782(98)00049-8. [DOI] [PubMed] [Google Scholar]
- 26.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 27.Haddad R, Guimiot F, Six E, Jourquin F, Setterblad N, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24(2):217–230. doi: 10.1016/j.immuni.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Miah M, Goh I, Haniffa M. Prenatal development and function of human mononuclear phagocytes. Front Cell Dev Biol. 2021;9:649937. doi: 10.3389/fcell.2021.649937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, et al. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity. 2019;50(6):1425–1438.e5. doi: 10.1016/j.immuni.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simic M, Manosalva I, Spinelli L, Gentek R, Shayan RR, et al. Distinct waves from the hemogenic endothelium give rise to layered lymphoid tissue inducer cell ontogeny. Cell Reports. 2020;32(6):108004. doi: 10.1016/j.celrep.2020.108004. [DOI] [PubMed] [Google Scholar]
- 32.Gentek R, Ghigo C, Hoeffel G, Jorquera A, Msallam R, et al. Epidermal γδ T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J Exp Med. 2018;215(12):2994–3005. doi: 10.1084/jem.20181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeffel G, Wang Y, Greter M, See P, Teo P, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun. 2016;7:ncomms11852. doi: 10.1038/ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guilliams M, Scott CL. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol. 2017;17(7):451–460. doi: 10.1038/nri.2017.42. [DOI] [PubMed] [Google Scholar]
- 43.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster CA, Holbrook KA, Farr AG. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol. 1986;86(3):240–243. doi: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- 45.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36(5):873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furio L, Briotet I, Journeaux A, Billard H, Péguet-Navarro J. Human langerhans cells are more efficient than CD14(-)CD1c(+) dermal dendritic cells at priming naive CD4(+) T cells. J Invest Dermatol. 2010;130(5):1345–1354. doi: 10.1038/jid.2009.424. [DOI] [PubMed] [Google Scholar]
- 47.Fujita M, Furukawa F, Horiguchi Y, Ueda M, Kashihara-Sawami M, Imamura S. Regional development of Langerhans cells and formation of Birbeck granules in human embryonic and fetal skin. J Invest Dermatol. 1991;97(1):65–72. doi: 10.1111/1523-1747.ep12478115. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371(6527):eaba6500. doi: 10.1126/science.aba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palis J. Interaction of the macrophage and primitive erythroid lineages in the mammalian embryo. Front Immunol. 2016;7:669. doi: 10.3389/fimmu.2016.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Wang Y, Zhao H, Zhang H, Xu Y, et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood. 2019;134(5):480–491. doi: 10.1182/blood.2019000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kutteh WH, Rainey WE, Carr BR. Glucocorticoids inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by human fetal Kupffer cells. J Clin Endocrinol Metab. 1991;73(2):296–301. doi: 10.1210/jcem-73-2-296. [DOI] [PubMed] [Google Scholar]
- 52.Kutteh WH, Rainey WE, Carr BR. Regulation of interleukin-6 production in human fetal Kupffer cells. Scand J Immunol. 1991;33(5):607–613. doi: 10.1111/j.1365-3083.1991.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 53.Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39(4):350–364. doi: 10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Okreglicka K, Iten I, Pohlmeier L, Onder L, Feng Q, et al. PPARγ is essential for the development of bone marrow erythroblastic island macrophages and splenic red pulp macrophages. J Exp Med. 2021;218(5):e20191314. doi: 10.1084/jem.20191314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurotaki D, Uede T, Tamura T. Functions and development of red pulp macrophages. Microbiol Immunol. 2015;59(2):55–62. doi: 10.1111/1348-0421.12228. [DOI] [PubMed] [Google Scholar]
- 56.Dege C, Fegan KH, Creamer JP, Berrien-Elliott MM, Luff SA, et al. Potently cytotoxic natural killer cells initially emerge from erythro-myeloid progenitors during mammalian development. Dev Cell. 2020;53(2):229–239.e7. doi: 10.1016/j.devcel.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Pavert SA. Lymphoid tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. Biomed J. 2021;44(2):123–132. doi: 10.1016/j.bj.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McVay LD, Jaswal SS, Kennedy C, Hayday A, Carding SR. The generation of human gammadelta T cell repertoires during fetal development. J Immunol. 1998;160(12):5851–5860. [PubMed] [Google Scholar]
- 60.Beaudin AE, Boyer SW, Perez-Cunningham J, Hernandez GE, Derderian SC, et al. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19(6):768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7(3):213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 62.Bueno C, van Roon EHJ, Muñoz-López A, Sanjuan-Pla A, Juan M, et al. Immunophenotypic analysis and quantification of B-1 and B-2 B cells during human fetal hematopoietic development. Leukemia. 2016;30(7):1603–1606. doi: 10.1038/leu.2015.362. [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Gong Y, Zhang H, Yang H, Zeng Y, et al. Delineating spatiotemporal and hierarchical development of human fetal innate lymphoid cells. Cell Res. 2021;31(10):1106–1122. doi: 10.1038/s41422-021-00529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330(6011):1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng MSF, Roth TL, Mendoza VF, Marson A, Burt TD. Helios enhances the preferential differentiation of human fetal CD4+ naïve T cells into regulatory T cells. Sci Immunol. 2019;4(41):eaav5947. doi: 10.1126/sciimmunol.aav5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li N, van Unen V, Abdelaal T, Guo N, Kasatskaya SA, et al. Memory CD4+ T cells are generated in the human fetal intestine. Nat Immunol. 2019;20(3):301–312. doi: 10.1038/s41590-018-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rackaityte E, Halkias J. Mechanisms of fetal T cell tolerance and immune regulation. Front Immunol. 2020;11:588. doi: 10.3389/fimmu.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haynes BF, Martin ME, Kay HH, Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988;168(3):1061–80. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35(2):383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- 71.Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176(10):5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 72.Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, et al. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc Natl Acad Sci U S A. 2015;112(6):E556–565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reitermaier R, Krausgruber T, Fortelny N, Ayub T, Vieyra-Garcia PA, et al. αβγδ T cells play a vital role in fetal human skin development and immunity. J Exp Med. 2021;218(4):e20201189. doi: 10.1084/jem.20201189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halkias J, Rackaityte E, Hillman SL, Aran D, Mendoza VF, et al. CD161 contributes to prenatal immune suppression of IFNγ-producing PLZF+ T cells. J Clin Invest. 2019;129(9):3562–3577. doi: 10.1172/JCI125957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darrasse-Jèze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105(12):4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 76.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 77.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol Blood Marrow Transplant. 2006;12(2):160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 78.Fawkner-Corbett D, Antanaviciute A, Parikh K, Jagielowicz M, Gerós AS, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184(3):810–826.e23. doi: 10.1016/j.cell.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol. 2017;10(1):5–17. doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 80.Li N, van Unen V, Guo N, Abdelaal T, Somarakis A, et al. Early-life compartmentalization of immune cells in human fetal tissues revealed by high-dimensional mass cytometry. Front Immunol. 2019;10:1932. doi: 10.3389/fimmu.2019.01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li N, van Unen V, Höllt T, Thompson A, van Bergen J, et al. Mass cytometry reveals innate lymphoid cell differentiation pathways in the human fetal intestine. J Exp Med. 2018;215(5):1383–1396. doi: 10.1084/jem.20171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stras SF, Werner L, Toothaker JM, Olaloye OO, Oldham AL, et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev Cell. 2019;51(3):357–373.e5. doi: 10.1016/j.devcel.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Blümer N, Pfefferle PI, Renz H. Development of mucosal immune function in the intrauterine and early postnatal environment. Curr Opin Gastroenterol. 2007;23(6):655–660. doi: 10.1097/MOG.0b013e3282eeb428. [DOI] [PubMed] [Google Scholar]
- 84.O’Connell A, Rivers A, Slayton W (2021) The development of the human immune system. In: De Alarcón P, Werner E, Christensen R, Sola-Visner M (eds) Neonatal hematology: pathogenesis, diagnosis, and management of hematologic problems. Cambridge University Press, Cambridge, pp 25–42. 10.1017/9781108773584.005
- 85.MacDonald TT, Spencer J. Ontogeny of the gut-associated lymphoid system in man. Acta Paediatr Suppl. 1994;83(395):3–5. doi: 10.1111/j.1651-2227.1994.tb13219.x. [DOI] [PubMed] [Google Scholar]
- 86.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 87.Dogra P, Rancan C, Ma W, Toth M, Senda T, et al. Tissue determinants of human NK cell development, function, and residence. Cell. 2020;180(4):749–763.e13. doi: 10.1016/j.cell.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howie D, Spencer J, DeLord D, Pitzalis C, Wathen NC, et al. Extrathymic T cell differentiation in the human intestine early in life. J Immunol. 1998;161(11):5862–5872. [PubMed] [Google Scholar]
- 89.Elmentaite R, Kumasaka N, Roberts K, Fleming A, Dann E, et al. Cells of the human intestinal tract mapped across space and time. Nature. 2021;597(7875):250–255. doi: 10.1038/s41586-021-03852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.James KR, Elmentaite R, Teichmann SA, Hold GL (2022) Redefining intestinal immunity with single-cell transcriptomics. Mucosal Immunol 30:1–11. 10.1038/s41385-021-00470-y [DOI] [PMC free article] [PubMed]
- 91.Krishnamurty AT, Turley SJ. Lymph node stromal cells: cartographers of the immune system. Nat Immunol. 2020;21(4):369–380. doi: 10.1038/s41590-020-0635-3. [DOI] [PubMed] [Google Scholar]
- 92.Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, et al. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G1–10. doi: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80(3):492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 94.Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120(22):4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 95.Mishra A, Lai GC, Yao LJ, Aung TT, Shental N, et al. Microbial exposure during early human development primes fetal immune cells. Cell. 2021;184(13):3394–3409.e20. doi: 10.1016/j.cell.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schreurs RRCE, Sagebiel AF, Steinert FL, Highton AJ, Klarenbeek PL, et al. Intestinal CD8+ T cell responses are abundantly induced early in human development but show impaired cytotoxic effector capacities. Mucosal Immunol. 2021;14(3):605–614. doi: 10.1038/s41385-021-00382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, et al. Developmental origin governs CD8+ T Cell fate decisions during infection. Cell. 2018;174(1):117–130.e14. doi: 10.1016/j.cell.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 98.Loh L, Ivarsson MA, Michaëlsson J, Sandberg JK, Nixon DF. Invariant natural killer T cells developing in the human fetus accumulate and mature in the small intestine. Mucosal Immunol. 2014;7(5):1233–1243. doi: 10.1038/mi.2014.13. [DOI] [PubMed] [Google Scholar]
- 99.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grassi R, Farina R, Floriani I, Amodio F, Romano S. Assessment of fetal swallowing with gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2005;185(5):1322–1327. doi: 10.2214/AJR.04.1114. [DOI] [PubMed] [Google Scholar]
- 101.Diamant NE. Development of esophageal function. Am Rev Respir Dis. 1985;131(5):S29–32. doi: 10.1164/arrd.1985.131.S5.S29. [DOI] [PubMed] [Google Scholar]
- 102.Kennedy KM, Bellissimo CJ, Breznik JA, Barrett J, Braun T, et al. Over-celling fetal microbial exposure. Cell. 2021;184(24):5839–5841. doi: 10.1016/j.cell.2021.10.026. [DOI] [PubMed] [Google Scholar]
- 103.Mishra A, Yao LJ, Wasser M, Khyriem C, Malleret B, et al. Reply to Over-celling fetal microbial exposure. Cell. 2021;184(24):5842–5844. doi: 10.1016/j.cell.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 104.Walter J, Hornef MW. A philosophical perspective on the prenatal in utero microbiome debate. Microbiome. 2021;9(1):5. doi: 10.1186/s40168-020-00979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 106.Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, et al. Innate immunity of the lung: from basic mechanisms to translational medicine. J Innate Immun. 2018;10(5–6):487–501. doi: 10.1159/000487057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joshi S, Kotecha S. Lung growth and development. Early Hum Dev. 2007;83(12):789–794. doi: 10.1016/j.earlhumdev.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 108.Dame JB, Christensen RD, Juul SE. The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr Res. 1999;46(4):358–366. doi: 10.1203/00006450-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 109.Marquardt N, Ivarsson MA, Sundström E, Åkesson E, Martini E, et al. Fetal CD103 + IL-17–producing group 3 innate lymphoid cells represent the dominant lymphocyte subset in human amniotic fluid. J Immunol. 2016;197(8):3069–75. doi: 10.4049/jimmunol.1502204. [DOI] [PubMed] [Google Scholar]
- 110.McGovern N, Shin A, Low G, Low D, Duan K, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature. 2017;546(7660):662–666. doi: 10.1038/nature22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5(1):16. doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- 112.Hou F, Xiao K, Tang L, Xie L. Diversity of macrophages in lung homeostasis and diseases. Front Immunol. 2021;12:753940. doi: 10.3389/fimmu.2021.753940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5(6):605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 114.Lakhdari O, Yamamura A, Hernandez GE, Anderson KK, Lund SJ, et al. Differential immune activation in fetal macrophage populations. Sci Rep. 2019;9(1):7677. doi: 10.1038/s41598-019-44181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15(11):1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 116.van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity. 2016;44(4):755–768. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Evren E, Ringqvist E, Doisne J-M, Thaller A, Sleiers N, et al. CD116+ fetal precursors migrate to the perinatal lung and give rise to human alveolar macrophages. J Experiment Med. 2022;219(2):e20210987. doi: 10.1084/jem.20210987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Phillips JH, Hori T, Nagler A, Bhat N, Spits H, Lanier LL. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon, delta proteins. J Exp Med. 1992;175(4):1055–1066. doi: 10.1084/jem.175.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan JKH, Watanabe T. Murine spleen tissue regeneration from neonatal spleen capsule requires lymphotoxin priming of stromal cells. J.I. 2014;193(3):1194–1203. doi: 10.4049/jimmunol.1302115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eberl G, Marmon S, Sunshine M-J, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, et al. IL-7 receptor α+ CD3– cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11(5):643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 122.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 123.Yu JC, Khodadadi H, Malik A, Davidson B, da Salles É, SL, , et al. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saluzzo S, Gorki A-D, Rana BMJ, Martins R, Scanlon S, et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep. 2017;18(8):1893–1905. doi: 10.1016/j.celrep.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11(1):171. doi: 10.1186/1479-5876-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4(10):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rolandsson Enes S, Andersson Sjöland A, Skog I, Hansson L, Larsson H, et al. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci Rep. 2016;6(1):29160. doi: 10.1038/srep29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kevill KA, Bhandari V, Kettunen M, Leng L, Fan J, et al. A role for macrophage migration inhibitory factor in the neonatal respiratory distress syndrome. J Immunol. 2008;180(1):601–608. doi: 10.4049/jimmunol.180.1.601. [DOI] [PubMed] [Google Scholar]
- 129.Perveen S, Ayasolla K, Zagloul N, Patel H, Ochani K, et al. MIF inhibition enhances pulmonary angiogenesis and lung development in congenital diaphragmatic hernia. Pediatr Res. 2019;85(5):711–718. doi: 10.1038/s41390-019-0335-6. [DOI] [PubMed] [Google Scholar]
- 130.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016;89(2):248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016.e27. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol. 2021;22(9):1083–1092. doi: 10.1038/s41590-021-00994-2. [DOI] [PubMed] [Google Scholar]
- 133.Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373(6553):eabf7844. doi: 10.1126/science.abf7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci. 2017;20(9):1300–1309. doi: 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- 135.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol. 2017;17(3):179–194. doi: 10.1038/nri.2016.144. [DOI] [PubMed] [Google Scholar]
- 137.Urban SL, Jensen IJ, Shan Q, Pewe LL, Xue H-H, et al. Peripherally induced brain tissue-resident memory CD8+ T cells mediate protection against CNS infection. Nat Immunol. 2020;21(8):938–949. doi: 10.1038/s41590-020-0711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48(2):380–395.e6. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 139.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci. 2016;10:566. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16(3):273–80. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 142.Goldmann T, Wieghofer P, Jordão MJC, Prutek F, Hagemeyer N, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang CC, Wu CH, Shieh JY, Wen CY. Microglial distribution and apoptosis in fetal rat brain. Brain Res Dev Brain Res. 2002;139(2):337–342. doi: 10.1016/S0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- 144.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 145.Kim I, Mlsna LM, Yoon S, Le B, Yu S, et al. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav. 2015;5(12):e00403. doi: 10.1002/brb3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol. 2015;278:280–288. doi: 10.1016/j.jneuroim.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cuadros MA, Navascués J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56(2):173–189. doi: 10.1016/S0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 148.Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814(1–2):13–25. doi: 10.1016/S0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- 149.Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499(4):565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- 150.Monier A, Adle-Biassette H, Delezoide A-L, Evrard P, Gressens P, Verney C. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol. 2007;66(5):372–382. doi: 10.1097/nen.0b013e3180517b46. [DOI] [PubMed] [Google Scholar]
- 151.Male D, Rezaie P. Colonisation of the human central nervous system by microglia: the roles of chemokines and vascular adhesion molecules. Prog Brain Res. 2001;132:81–93. doi: 10.1016/S0079-6123(01)32067-8. [DOI] [PubMed] [Google Scholar]
- 152.Menassa DA, Gomez-Nicola D. Microglial dynamics during human brain development. Front Immunol. 2018;9:1014. doi: 10.3389/fimmu.2018.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–547. doi: 10.1016/S0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 154.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790. doi: 10.3389/fimmu.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33(10):4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 159.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 161.Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34(6):2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 163.Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33(3):586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 164.Priego N, Valiente M. The potential of astrocytes as immune modulators in brain tumors. Front Immunol. 2019;10:1314. doi: 10.3389/fimmu.2019.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150(7):2659–67. [PubMed] [Google Scholar]
- 166.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 167.Holst CB, Brøchner CB, Vitting-Seerup K, Møllgård K. Astrogliogenesis in human fetal brain: complex spatiotemporal immunoreactivity patterns of GFAP, S100, AQP4 and YKL-40. J Anat. 2019;235(3):590–615. doi: 10.1111/joa.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kadhim HJ, Gadisseux JF, Evrard P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: histochemical and electron microscopic study. J Neuropathol Exp Neurol. 1988;47(2):166–188. doi: 10.1097/00005072-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 169.Marín-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol. 1995;357(4):554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- 170.Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359(6381):1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508(5):687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 173.Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell. 2020;182(3):625–640.e24. doi: 10.1016/j.cell.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tanabe S, Yamashita T. B-1a lymphocytes promote oligodendrogenesis during brain development. Nat Neurosci. 2018;21(4):506–516. doi: 10.1038/s41593-018-0106-4. [DOI] [PubMed] [Google Scholar]
- 175.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 176.Cui J, Xu H, Lehtinen MK. Macrophages on the margin: choroid plexus immune responses. Trends Neurosci. 2021;44(11):864–875. doi: 10.1016/j.tins.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cui J, Shipley FB, Shannon ML, Alturkistani O, Dani N, et al. Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Dev Cell. 2020;55(5):617–628.e6. doi: 10.1016/j.devcel.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–3074.e21. doi: 10.1016/j.cell.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lenz KM, Pickett LA, Wright CL, Davis KT, Joshi A, McCarthy MM. Mast cells in the developing brain determine adult sexual behavior. J Neurosci. 2018;38(37):8044–8059. doi: 10.1523/JNEUROSCI.1176-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lim AI, McFadden T, Link VM, Han S-J, Karlsson R-M, et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science. 2021;373(6558):eabf3002. doi: 10.1126/science.abf3002. [DOI] [PubMed] [Google Scholar]
- 181.Nyangahu DD, Jaspan HB. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. 2019;198(1):47–56. doi: 10.1111/cei.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stinson LF, Boyce MC, Payne MS, Keelan JA. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol. 2019;10:1124. doi: 10.3389/fmicb.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dimova T, Terzieva A, Djerov L, Dimitrova V, Nikolov A, et al. Mother-to-newborn transmission of mycobacterial L-forms and Vδ2 T-cell response in placentobiome of BCG-vaccinated pregnant women. Sci Rep. 2017;7(1):17366. doi: 10.1038/s41598-017-17644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jennewein MF, Goldfarb I, Dolatshahi S, Cosgrove C, Noelette FJ, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell. 2019;178(1):202–215.e14. doi: 10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Albrecht M, Pagenkemper M, Wiessner C, Spohn M, Lütgehetmann M, et al. Infant immunity against viral infections is advanced by the placenta-dependent vertical transfer of maternal antibodies. Vaccine. 2021;S0264–41X0(20):31629–7. doi: 10.1016/j.vaccine.2020.12.049. [DOI] [PubMed] [Google Scholar]
- 186.Msallam R, Balla J, Rathore APS, Kared H, Malleret B, et al. Fetal mast cells mediate postnatal allergic responses dependent on maternal IgE. Science. 2020;370(6519):941–950. doi: 10.1126/science.aba0864. [DOI] [PubMed] [Google Scholar]
- 187.Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21(5):561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hoo R, Nakimuli A, Vento-Tormo R. Innate immune mechanisms to protect against infection at the human decidual-placental interface. Front Immunol. 2020;11:2070. doi: 10.3389/fimmu.2020.02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol. 2017;17(8):483–494. doi: 10.1038/nri.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stelzer IA, Urbschat C, Schepanski S, Thiele K, Triviai I, et al. Vertically transferred maternal immune cells promote neonatal immunity against early life infections. Nat Commun. 2021;12(1):4706. doi: 10.1038/s41467-021-24719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Juber BA, Elgin TG, Fricke EM, Gong H, Reese J, McElroy SJ (2020) A murine model of fetal exposure to maternal inflammation to study the effects of acute chorioamnionitis on newborn intestinal development. J Vis Exp (160). 10.3791/61464 [DOI] [PubMed]
- 192.Elgin TG, Fricke EM, Gong H, Reese J, Mills DA, et al. Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury. Dis Model Mech. 2019;12(10):dmm040808. doi: 10.1242/dmm.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jackson CM, Mukherjee S, Wilburn AN, Cates C, Lewkowich IP, et al. Pulmonary consequences of prenatal inflammatory exposures: clinical perspective and review of basic immunological mechanisms. Front Immunol. 2020;11:1285. doi: 10.3389/fimmu.2020.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Singh AM, Sherenian MG, Kim K-Y, Erickson KA, Yang A, et al. Fetal cord blood and tissue immune responses to chronic placental inflammation and chorioamnionitis. Allergy Asthma Clin Immunol. 2018;14:66. doi: 10.1186/s13223-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14(1):2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection - maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299(Pt A):241–251. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choudhury Z, Lennox B. Maternal immune activation and schizophrenia-evidence for an immune priming disorder. Front Psychiatry. 2021;12:585742. doi: 10.3389/fpsyt.2021.585742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jones HF, Han VX, Patel S, Gloss BS, Soler N, et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. 2021;94:308–317. doi: 10.1016/j.bbi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 199.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 2018;21(5):765–772. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ganguli S, Chavali PL. Intrauterine viral infections: impact of inflammation on fetal neurodevelopment. Front Neurosci. 2021;15:771557. doi: 10.3389/fnins.2021.771557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tong L, Kalish BT. The impact of maternal obesity on childhood neurodevelopment. J Perinatol. 2021;41(5):928–939. doi: 10.1038/s41372-020-00871-0. [DOI] [PubMed] [Google Scholar]
- 202.Lautarescu A, Craig MC, Glover V. Prenatal stress: effects on fetal and child brain development. Int Rev Neurobiol. 2020;150:17–40. doi: 10.1016/bs.irn.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 203.Fruntes V, Limosin F. Schizophrenia and viral infection during neurodevelopment: a pathogenesis model? Med Sci Monit. 2008;14(6):RA71–77. [PubMed] [Google Scholar]
- 204.Selten J-P, Termorshuizen F. The serological evidence for maternal influenza as risk factor for psychosis in offspring is insufficient: critical review and meta-analysis. Schizophr Res. 2017;183:2–9. doi: 10.1016/j.schres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 205.Rathore APS, Saron WAA, Lim T, Jahan N, St John AL. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv. 2019;5(2):eaav3208. doi: 10.1126/sciadv.aav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thion MS, Low D, Silvin A, Chen J, Grisel P, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell. 2018;172(3):500–516.e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 208.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25(35):8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mirabella F, Desiato G, Mancinelli S, Fossati G, Rasile M, et al. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity. 2021;54(11):2611–2631.e8. doi: 10.1016/j.immuni.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ratnayake U, Quinn T, Walker DW, Dickinson H. Cytokines and the neurodevelopmental basis of mental illness. Front Neurosci. 2013;7:180. doi: 10.3389/fnins.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Saunders NR, Dziegielewska KM, Møllgård K, Habgood MD. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J Physiol. 2018;596(23):5723–5756. doi: 10.1113/JP275376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schmitt C, Strazielle N, Ghersi-Egea J-F. Brain leukocyte infiltration initiated by peripheral inflammation or experimental autoimmune encephalomyelitis occurs through pathways connected to the CSF-filled compartments of the forebrain and midbrain. J Neuroinflammation. 2012;9:187. doi: 10.1186/1742-2094-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kowal C, Athanassiou A, Chen H, Diamond B. Maternal antibodies and developing blood-brain barrier. Immunol Res. 2015;63(1–3):18–25. doi: 10.1007/s12026-015-8714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Werner Y, Mass E, Ashok Kumar P, Ulas T, Händler K, et al. Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat Neurosci. 2020;23(3):351–362. doi: 10.1038/s41593-020-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Herz J, Bendix I, Felderhoff-Müser U (2022) Peripheral immune cells and perinatal brain injury: a double-edged sword? Pediatr Res 91:392–403 [DOI] [PMC free article] [PubMed]
- 218.Melo AM, Taher NA, Doherty DG, Molloy EJ. The role of lymphocytes in neonatal encephalopathy. Brain Behav Immun Health. 2021;18:100380. doi: 10.1016/j.bbih.2021.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Albertsson A-M, Zhang X, Vontell R, Bi D, Bronson RT, et al. γδ T cells contribute to injury in the developing brain. Am J Pathol. 2018;188(3):757–767. doi: 10.1016/j.ajpath.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Seki D, Mayer M, Hausmann B, Pjevac P, Giordano V, et al. Aberrant gut-microbiota-immune-brain axis development in premature neonates with brain damage. Cell Host Microbe. 2021;29(10):1558–1572.e6. doi: 10.1016/j.chom.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lewis EL, Tulina N, Anton L, Brown AG, Porrett PM, Elovitz MA. IFNγ-producing γ/δ T cells accumulate in the fetal brain following intrauterine inflammation. Front Immunol. 2021;12:741518. doi: 10.3389/fimmu.2021.741518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, et al. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Natama HM, Moncunill G, Rovira-Vallbona E, Sanz H, Sorgho H, et al. Modulation of innate immune responses at birth by prenatal malaria exposure and association with malaria risk during the first year of life. BMC Med. 2018;16(1):198. doi: 10.1186/s12916-018-1187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abu-Raya B, Kollmann TR, Marchant A, MacGillivray DM. The immune system of HIV-exposed uninfected infants. Front Immunol. 2016;7:383. doi: 10.3389/fimmu.2016.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mincham KT, Jones AC, Bodinier M, Scott NM, Lauzon-Joset J-F, et al. Transplacental innate immune training via maternal microbial exposure: role of XBP1-ERN1 axis in dendritic cell precursor programming. Front Immunol. 2020;11:601494. doi: 10.3389/fimmu.2020.601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12(4):330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 228.Kollmann TR, Marchant A, Way SS. Vaccination strategies to enhance immunity in neonates. Science. 2020;368(6491):612–615. doi: 10.1126/science.aaz9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tieppo P, Papadopoulou M, Gatti D, McGovern N, Chan JKY, et al. The human fetal thymus generates invariant effector γδ T cells. J Exp Med. 2020;217(3):e20190580. doi: 10.1084/jem.20190580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee YJ, Jeon YK, Kang BH, Chung DH, Park C-G, et al. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207(1):237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Galindo-Albarrán AO, López-Portales OH, Gutiérrez-Reyna DY, Rodríguez-Jorge O, Sánchez-Villanueva JA, et al. CD8+ T cells from human neonates are biased toward an innate immune response. Cell Rep. 2016;17(8):2151–2160. doi: 10.1016/j.celrep.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 232.Kinder JM, Jiang TT, Ertelt JM, Xin L, Strong BS, et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell. 2015;162(3):505–515. doi: 10.1016/j.cell.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schuster C, Vaculik C, Prior M, Fiala C, Mildner M, et al. Phenotypic characterization of leukocytes in prenatal human dermis. J Invest Dermatol. 2012;132(11):2581–2592. doi: 10.1038/jid.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111(11):1747–1755. doi: 10.1172/JCI200317470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Legrand FA, Nixon DF, Loo CP, Ono E, Chapman JM, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186(5):2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 237.Pietrzak-Nguyen A, Piradashvili K, Fichter M, Pretsch L, Zepp F, et al. MPLA-coated hepatitis B virus surface antigen (HBsAg) nanocapsules induce vigorous T cell responses in cord blood derived human T cells. Nanomedicine. 2016;12(8):2383–2394. doi: 10.1016/j.nano.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 238.Rastogi D, Wang C, Mao X, Lendor C, Rothman PB, Miller RL. Antigen-specific immune responses to influenza vaccine in utero. J Clin Invest. 2007;117(6):1637–1646. doi: 10.1172/JCI29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hendriks D, Artegiani B, Hu H, de Sousa C, Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat Protoc. 2021;16(1):182–217. doi: 10.1038/s41596-020-00411-2. [DOI] [PubMed] [Google Scholar]
- 240.Roodsant T, Navis M, Aknouch I, Renes IB, van Elburg RM, et al. A human 2D primary organoid-derived epithelial monolayer model to study host-pathogen interaction in the small intestine. Front Cell Infect Microbiol. 2020;10:272. doi: 10.3389/fcimb.2020.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pașca AM, Park J-Y, Shin H-W, Qi Q, Revah O, et al. Human 3D cellular model of hypoxic brain injury of prematurity. Nat Med. 2019;25(5):784–791. doi: 10.1038/s41591-019-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14(2):518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lewis K, Yoshimoto M, Takebe T. Fetal liver hematopoiesis: from development to delivery. Stem Cell Res Ther. 2021;12(1):139. doi: 10.1186/s13287-021-02189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Elahi S, Vega-López MA, Herman-Miguel V, Ramírez-Estudillo C, Mancilla-Ramírez J, et al. CD71+ erythroid cells in human neonates exhibit immunosuppressive properties and compromise immune response against systemic infection in neonatal mice. Front Immunol. 2020;11:597433. doi: 10.3389/fimmu.2020.597433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Delyea C, Bozorgmehr N, Koleva P, Dunsmore G, Shahbaz S, et al. CD71+ erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J Immunol. 2018;200(12):4044–4058. doi: 10.4049/jimmunol.1800113. [DOI] [PubMed] [Google Scholar]
- 246.Di Nuzzo S, Pavanello P, Masotti A, Giordano G, De Panfilis G. Densities, distribution and phenotypic expression of T cells in human fetal skin. Arch Dermatol Res. 2009;301(10):753–755. doi: 10.1007/s00403-009-0943-9. [DOI] [PubMed] [Google Scholar]
- 247.Kolter J, Feuerstein R, Zeis P, Hagemeyer N, Paterson N, et al. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity. 2019;50(6):1482–1497.e7. doi: 10.1016/j.immuni.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 248.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, et al. Spatiotemporal immune zonation of the human kidney. Science. 2019;365(6460):1461–1466. doi: 10.1126/science.aat5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308(1):232–246. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 250.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 251.Angelo LS, Bimler LH, Nikzad R, Aviles-Padilla K, Paust S. CXCR6+ NK cells in human fetal liver and spleen possess unique phenotypic and functional capabilities. Front Immunol. 2019;10:469. doi: 10.3389/fimmu.2019.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Antin JH, Emerson SG, Martin P, Gadol N, Ault KA. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986;136(2):505–10. [PubMed] [Google Scholar]
- 253.Park J-E, Botting RA, Domínguez Conde C, Popescu D-M, Lavaert M, et al. A cell atlas of human thymic development defines T cell repertoire formation. Science. 2020;367(6480):eaay3224. doi: 10.1126/science.aay3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zaharie D, Moleriu RD, Mic FA. Modeling the development of the post-natal mouse thymus in the absence of bone marrow progenitors. Sci Rep. 2016;6:36159. doi: 10.1038/srep36159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kernfeld EM, Genga RMJ, Neherin K, Magaletta ME, Xu P, Maehr R. A single-cell transcriptomic atlas of thymus organogenesis resolves cell types and developmental maturation. Immunity. 2018;48(6):1258–1270.e6. doi: 10.1016/j.immuni.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Stouch AN, McCoy AM, Greer RM, Lakhdari O, Yull FE, et al. IL-1β and inflammasome activity link inflammation to abnormal fetal airway development. J Immunol. 2016;196(8):3411–3420. doi: 10.4049/jimmunol.1500906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 258.Nishinakamura R, Wiler R, Dirksen U, Morikawa Y, Arai K, et al. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation. J Exp Med. 1996;183(6):2657–2662. doi: 10.1084/jem.183.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim HY, Lee HJ, Chang Y-J, Pichavant M, Shore SA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]