Substrate scope of 4-indolyl allylic carbonatesa.
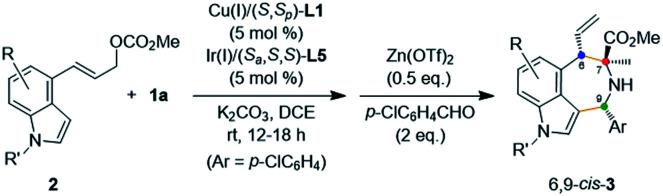
| ||||||
|---|---|---|---|---|---|---|
| Entry | R | R′ | 3 | drb | Yield (%)c | ee (%)d |
| 1 | 5-F | Me | 3x | 14 : 1 | 63 | 99 |
| 2 | 6-F | Me | 3y | 9 : 1 | 60 | 99 |
| 3 | 7-F | Me | 3z | 11 : 1 | 54 | 99 |
| 4 | 6-Br | Me | 3A | 12 : 1 | 63 | 99 |
| 5 | 6-Ph | Me | 3B | 20 : 1 | 56 | 99 |
| 6 | 6-vinyl | Me | 3C | 18 : 1 | 64 | 99 |
| 7 | 6-cyclopropyl | Me | 3D | 13 : 1 | 61 | 99 |
| 8 | H | Allyl | 3E | 10 : 1 | 63 | 99 |
| 9 | H | Bn | 3F | 15 : 1 | 61 | 97 |
| 10 | H | H | 3G | 11 : 1 | 66 | 99 |
All reactions were carried out with 0.30 mmol 1a and 0.20 mmol 2 in 2 mL of DCE. Cu(i) = Cu(MeCN)4BF4. Ir(i) = [Ir(cod)Cl]2.
dr was determined by the 1H NMR of the crude reaction mixture.
Isolated yields of the overall two steps.
ee was determined by chiral HPLC analysis.
