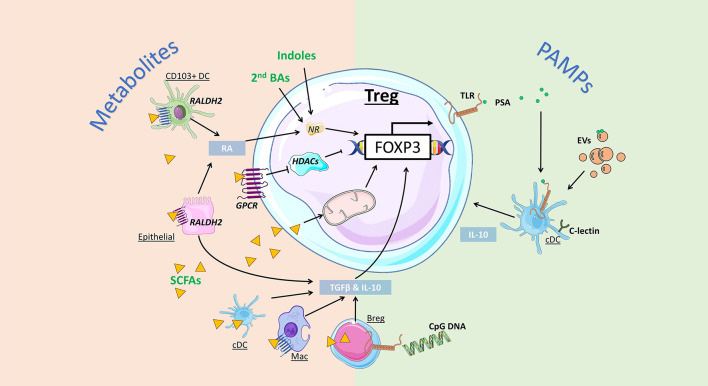
Figure 2.
Impact of gut bacteria on regulatory T cell development and function. Microbiota-derived metabolites can regulate Treg development and function via distinct mechanisms. Indoles and secondary bile acids (BAs) can bind to nuclear receptors (NRs) to promote Foxp3 expression and Treg induction. Short-chain fatty acids (SCFAs) can activate G protein-coupled receptors (GPCRs) to promote Treg directly via inhibition of histone deacetylase (HDAC) activity, or indirectly, by enhancing retinal dehydrogenases (RALDH) and retinoic acid (RA) production by CD103+ DCs and epithelial cells. Alternatively, SCFA are directly taken up by cells, including regulatory B cells (Bregs) and conventional DCs (cDCs) to promote expression of Treg-inducing cytokines TGF-β and IL-10. SCFA uptake by T cells can also directly promote Treg induction by increasing mitochondrial activity. Bacterial pathogen associated molecular patterns (PAMPs) can also promote Treg via activation of toll-like receptors (TLR). Bacteroides fragilis-derived polysaccharide A (PSA) promote Treg via TLR signalling on DCs, promoting IL-10 production, as well as Treg-intrinsic TLR2 signals. Extracellular vesicles (EVs) are PAMPs that can bind to TLR and C-lectin expressed by gut DCs and epithelial cells, triggering the release of Treg-inducing cytokines IL-10 and TGF-β.