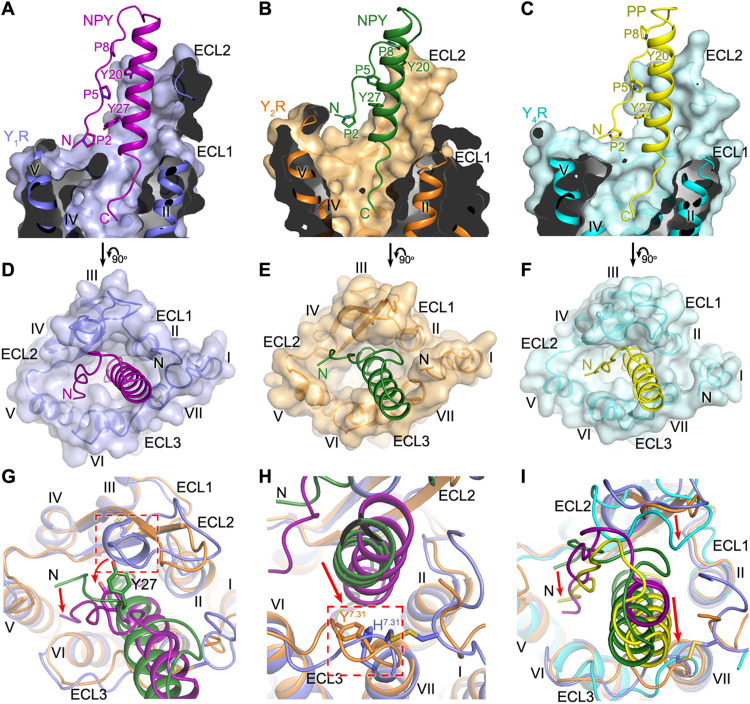
Fig. 2. Binding site of NPY peptides in YRs.
(A to C) Cutaway view of the NPY/PP-binding pocket in YRs. (A) The NPY-Y1R-Gi1 structure is shown in cartoon representation and colored blue (Y1R) and purple (NPY). The receptor is also shown as surface. (B) The NPY-Y2R-Gi1 structure is colored orange (Y2R) and green (NPY). (C) The PP-Y4R-Gi1 structure is colored cyan (Y4R) and yellow (PP). (D to F) Extracellular view of the NPY/PP-binding pocket in YRs. (D) NPY-Y1R-Gi1; (E) NPY-Y2R-Gi1; (F) PP-Y4R-Gi1. (G and H) Comparison of the binding pose of NPY at Y1R and Y2R. (G) The red arrows indicate the conformational changes of the peptide N terminus and the peptide residue Y27 in the NPY-Y1R-Gi1 structure relative to the NPY-Y2R-Gi1 structure. The extra one-turn helix in ECL2 of Y1R, which induces the movement of NPY toward ECL3, is highlighted by a red dashed box. (H) The red arrow indicates the movement of the α-helical region of NPY in the NPY-Y1R-Gi1 structure relative to the NPY-Y2R-Gi1 structure. The residues at position 7.31 (H7.31 in Y1R, Y7.31 in Y2R), which facilitate the movement of NPY, are highlighted by a red dashed box. (I) Comparison of the binding poses of NPY in Y1R/Y2R and PP in Y4R. The red arrows indicate the movements of the N terminus and α-helical region of PP and the receptor ECL2 in the PP-Y4R-Gi1 structure relative to the NPY-Y1R-Gi1 and NPY-Y2R-Gi1 structures.