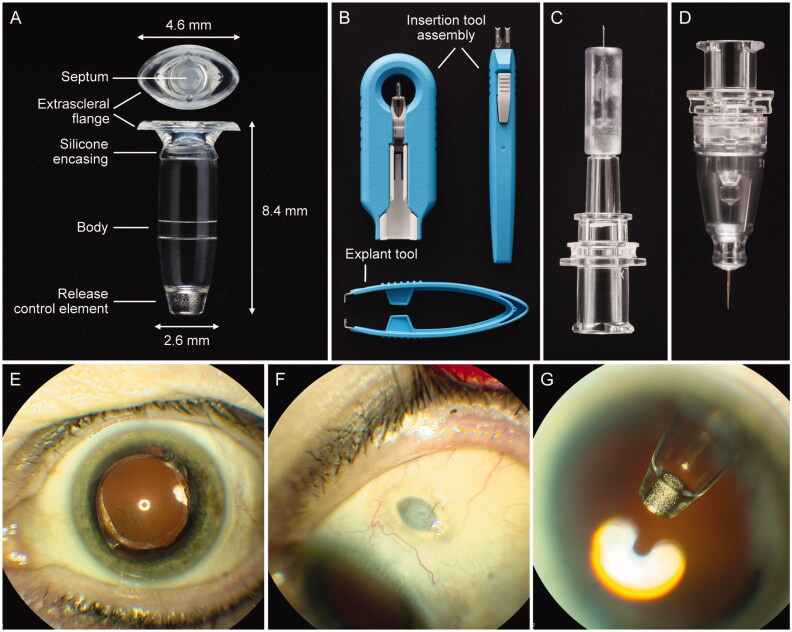
Figure 2.
The PDS ocular implant, ancillary devices, and position in situ. (A) PDS implant showing four key features: an extrascleral flange that anchors the implant in the sclera, a self-sealing septum that allows for implant refills, an implant body that contains the drug reservoir for the customized formulation of ranibizumab, and the porous titanium RCE that controls the rate of ranibizumab diffusion into the vitreous. (B) Insertion tool assembly to facilitate handling of the implant during the initial filling and insertion procedures, and explant tool (lower image) to grasp and securely hold the implant flange during the explant procedure (if explantation is clinically indicated). (C) Initial fill needle to fill the implant with ranibizumab before insertion. (D) Refill needle to refill-exchange, in situ, the implant with ranibizumab. (E–G) show the PDS implant in situ. Images from a PDS-implanted patient showing (E) eye in primary position (implant not visible), (F) eye looking down with the PDS implant septum visible, and (G) eye looking up with implant visible through dilated pupil. Copyright 2021 F. Hoffmann-La Roche Ltd., all rights reserved. Used with permission. Abbreviations: PDS: Port Delivery System with ranibizumab; RCE: release control element.