Highlights
-
•
Resistance training improved muscle strength but did not induce significant muscle hypertrophy.
-
•
There were no reported adverse events following resistance training in elderly cancer patients.
-
•
Muscle strength can be increased by resistance training at a moderate intensity for relatively short periods.
-
•
Resistance should be gradually increased throughout training period under the direction of practitioners.
Keywords: Cancer cachexia, Muscle synthesis, Sarcopenia, Skeletal muscle
Abstract
Background
One of the most life-threatening comorbidities in elderly cancer patients is cancer cachexia, which is characterized by the ongoing loss of skeletal muscular strength and mass and is also associated with aging. There is a lack of recommendations for optimal resistance training (RT) for those patients. The purpose of this study was to systematically review and quantify the effects of RT on muscular strength and hypertrophy in elderly cancer patients.
Methods
Five electronic databases were searched (until January 2020) for studies that met the following criteria: (i) cancer patients aged ≥60 years; (ii) structured and supervised RT intervention for ≥6 weeks; and (iii) measured muscular strength and/or hypertrophy.
Results
Thirteen studies (717 participants, average age = 66 years) met the inclusion criteria. RT significantly increased muscular strength (mean effect size = 0.87, 95% confidence interval (95%CI): 0.43–1.32, p < 0.001) and did not significantly induce muscle hypertrophy (mean effect size = 0.09, 95%CI: –0.14 to 0.31, p = 0.45). In subgroup analyses for muscle strength, higher weekly frequency was significantly associated with larger effect size. Egger's test showed no significant publication bias for the 2 outcomes.
Conclusion
The results suggest that RT improves muscular strength but does not significantly induce muscle hypertrophy in elderly cancer patients.
Graphical Abstract
1. Introduction
Elderly cancer patients account for 50% of all newly diagnosed cancer cases, and 71% of cancer deaths occur in those aged 65 years and older.1 One of the most life-threatening comorbidities in elderly cancer patients is cancer cachexia,2 a multifactorial syndrome characterized by the ongoing loss of skeletal muscular strength and mass, with or without the loss of fat mass.3 Cancer cachexia is caused by diminished caloric intake and abnormal metabolism induced by tumor-derived factors.4 Moreover, aging is closely associated with both decreased muscle strength and mass.5 Sarcopenia (degenerative loss of skeletal muscle mass, strength, and quality) usually starts after the age of 50,6 and the phenomenon worsens after age 60.7 Sarcopenia aggravates cancer cachexia8 and, consequently, augments cancer mortality in elderly populations.9,10 Thus, elderly cancer patients need to maintain or increase their muscle strength and mass to increase their chance of survival.
It has been well established that resistance training (RT) increases both muscular strength and mass in the general elderly population.5,11 RT induces neuromuscular adaptations through increased motoneuron recruitment and excitability, which improves muscle cell activation during voluntary muscle contractions.12 Cancer-related muscle atrophy can be attenuated by RT, which downregulates the ATP-dependent ubiquitin–proteasome system (a principal system responsible for muscle protein degradation in cancer) by decreasing the activity of proinflammatory cytokines.13
There are 2 previous meta-analysis studies which report that RT increases muscular strength and/or mass in cancer patients.14,15 However, the 2 existing studies investigated cancer patients who were mostly middle-aged or who were only undergoing neoadjuvant and adjuvant therapy14,15; furthermore, they lacked clear recommendations for optimal RT. Accordingly, the practical application of RT for elderly cancer patients is underdeveloped. Therefore, the primary purpose of this study was to evaluate the effects of RT on muscular strength and hypertrophy in elderly cancer patients by conducting a systematic review and meta-analysis. This review carefully examined the specific variables of the exercise training regimens (i.e., intensity, duration, frequency, and volume), different types of cancer populations (tumor type, type of cancer treatment received, and stage of cancer treatment), and reported adverse events in order to provide sufficient evidence for establishing optimal exercise guidelines. Moreover, it was hoped this study would reveal current bottlenecks in the field and inspire future research to resolve these issues.
2. Methods
This current systematic review followed the strategy of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) Statement.16 Ethics committee approval was not sought for the present study because this meta-analysis study was based on the results of previously published studies.
2.1. Data sources
Five electronic databases (CINAHL, EMBASE, PubMed, SportDiscus, and Web of Science) were searched for eligible studies published in English from the earliest date available to January 2020. The following keywords were used for searches: “resistance training or strength training or weight training”, “muscular strength or muscle hypertrophy or muscle”, and “cancer”. Manual searches of reference lists were conducted to ensure all relevant studies were captured. One reviewer (JL) searched all of the articles and applied the inclusion and exclusion criteria to the titles and abstracts searched. When the information was not clear, the full text papers of the studies were obtained for review. Corresponding authors of potentially eligible studies were contacted if studies reported data for which it was impossible to discriminate.
2.2. Study selection
The inclusion criteria for eligible studies were as follows: (i) cancer patients aged ≥60 years who have been actively receiving treatment or are in long-term follow-up; (ii) structured and supervised resistance exercise intervention for ≥6 weeks; and (iii) measured muscular strength and/or hypertrophy. Studies including additional interventions such as aerobic training or dietary supplements were excluded in order to focus on the effects of RT alone. Duplicate studies or sub-studies of included trials were also excluded from the analysis.
2.3. Quality assessment
One reviewer (JL) assessed the quality of the included studies using the PRISMA recommendations.16 The quality assessment consisted of 6 items: (i) appropriate generation of random allocation sequence; (ii) concealment of the allocation sequence; (iii) blinding of the assessment and collection outcomes; (iv) proportion of participants lost to follow-up; (v) complete outcome data; and (vi) the intention-to-treat principle.16
2.4. Data extraction
Data were extracted from all selected studies and recorded in detail with respect to subject characteristics, study methods, interventions, outcomes, and adverse events. We used mean and standard deviation (SD), and where the standard error (SE) or 95% confidence interval (95%CI) were provided, they were converted to SD.
Population characteristics, age, gender, body mass index (BMI), body fat percentage, and the number of participants were recorded from each study in order to compare the similarity of participants between trials. The primary outcomes were muscular strength (percent changes in the one-repetition maximum (1-RM) or dynamometry) and muscle hypertrophy (lean body mass or cross-sectional area (CSA)). We selected only whole-body lean mass or whole-body fat-free mass for muscle mass. If muscular strength or CSA were reported as upper and lower body, we used the lower body or dominant leg. To compare the similarity of training methods between trials, we recorded for each intervention the total duration, frequency (days per week), intensity, repetition, set, rest periods, session duration, type and order of exercise, names of exercise machine or tool, supervisors, places of intervention, and whether exercise was performed to muscular fatigue or not. The methods of measuring muscular strength and/or mass were also extracted. The median values were used for calculation if the studies reported a range of data (e.g., 16 of repetitions was used to report 15–17 of repetitions). Detailed interventions about control groups (CON) and any additional interventions were recorded.
2.5. Data analysis
Heterogeneity between studies was assessed using the Cochran Q statistic17 and the I2 test.18 I2 ranges from 0 to 100%: a value <25% indicates a low risk of heterogeneity, 25%–75% indicates a moderate risk of heterogeneity, and >75% indicates a high risk of heterogeneity. In each study, the effect size (ES) for the intervention was calculated by the standardized mean difference between pre- and post-intervention using Cohen's d. Separate meta-analyses of trials with muscular strength and hypertrophy were performed to generate the mean ES and 95%CI. ESs were classified according to Cohen's definition,19 where 0.2 is considered small, 0.5 moderate, and 0.8 large. We used a fixed-effects model when homogeneity was verified or a random-effects model when heterogeneity was shown by the Q statistic.18 When the Q value exceeds the degrees of freedom (df) of the estimate, significant heterogeneity is supposed to exist. Publication bias was assessed using the Egger's regression test.20 To evaluate whether an individual cohort had undue influence on the overall meta-analysis result, we performed sensitivity analyses on both outcomes by omitting 1 trial at a time and determining whether statistical conclusions remained the same. The percent changes in muscle strength and mass between pre- and post-intervention were calculated using the weighted averages. All calculations were conducted with SPSS Version 20.0 (IBM Corp., Armonk, NY, USA), Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA), and STATA Version 14.2 (Stata Corp., College Station, TX, USA).
Subgroup analyses were performed where sufficient numbers of trials existed in subgroups to identify factors that could potentially influence the effect of exercise on outcomes and account for the heterogeneity between studies: (i) male vs. female; (ii) <24 weeks vs. ≥24 weeks; (iii) 2 days per week vs. 3 days per week; (iv) moderate-intensity (50%–70% of 1-RM) vs. high-intensity (70%–90% of 1-RM); (v) <60 sets per week vs. ≥60 sets per week; and (vi) <540 repetitions per week vs. ≥540 repetitions per week. Random effects meta-analysis regression was conducted to compare the effect estimates in different subgroups by considering the meta-analysis results from each subgroup separately. To interpret the results of subgroup analyses, p < 0.05 between study variations was considered as the statistical difference between subgroups.
3. Results
3.1. Study selection and characteristics
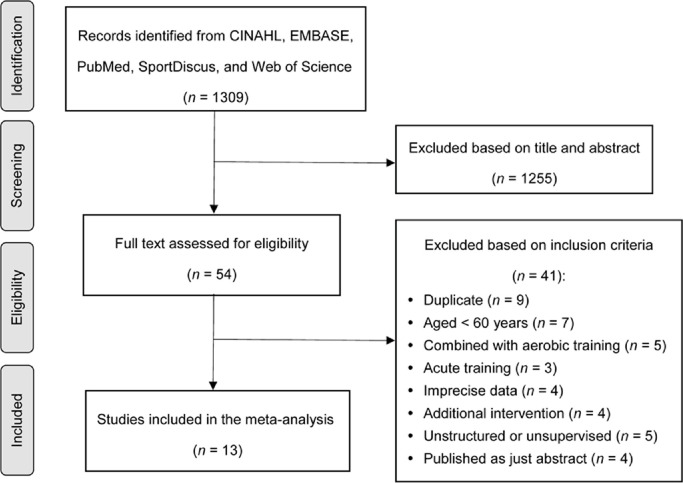
The selection process resulted in 1309 potential studies and was documented in the PRISMA flow diagram (Fig. 1). From the titles and abstracts, 1255 studies were excluded based on the inclusion and exclusion criteria, and then 54 full-text studies were reviewed for eligibility by applying the same criteria. Of these, 41 studies were excluded after assessment and, finally, a total of 13 articles met the criteria. In selected studies, 2 aerobic training groups21, 22 and 1 middle aged RT group (40–59 years)23 were excluded from our analysis. Consequently, 13 RT cohorts in 13 studies21−33 were selected.
Fig. 1.
Study search and selection process.
3.2. Quality assessment and potential bias
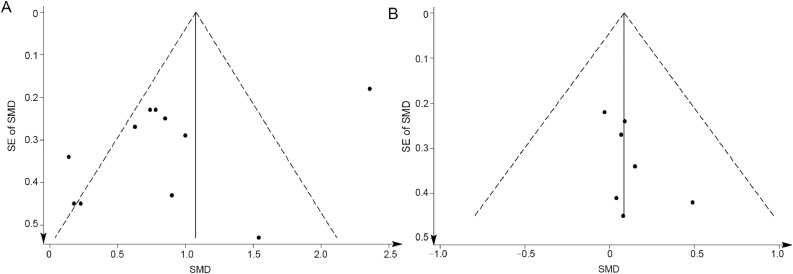
In the quality assessment, 10 studies22−25,27,28,30−33 reported appropriate generation of a random allocation sequence (77%), 4 studies24,26,29,33 presented concealment of the allocation sequence (31%), 7 studies21,23,24,26,29,32,33 described blinding of the assessment and collection outcomes (54%), all 13 studies explained proportion of participants lost to follow-up, all 13 studies exhibited complete outcome data, and 5 studies23,25,27,30,33 reported that the intention-to-treat principle (39%) were used for statistical analyses. Egger's test showed no significant publication bias for both muscular strength and hypertrophy (p = 0.08 and p = 0.17, respectively) (Fig. 2).
Fig. 2.
Funnel plots of publication bias in 2 outcomes: (A) muscular strength and (B) muscular hypertrophy. SE = standard error; SMD = standardized mean difference.
3.3. Adverse events
The presence or absence of adverse events was recorded in 10 studies of the 13 studies. Eight studies reported that there were no adverse events.23,26−32 One study reported that 3 patients discontinued the intervention due to knee or back pain.24 Another study reported that 1 patient experienced chest pain during exercise and subsequent cardiologic investigations were negative.22
3.4. Participants
Table 1 shows the characteristics of all of the studies included. Articles were published from May 200325 to June 2017.26 A total of 717 participants completed their interventions (RT = 394, CON = 323; females = 33.5%); study cohorts ranged from 8 participants23 to 155 participants.25 The average age of the participants was 66.0 ± 7.3 years (RT: 66.4 ± 7.5 years; CON: 65.5 ± 7.0 years; mean ± SD). BMI was 28.7 ± 4.3 kg/m2 (RT: 28.6 ± 4.3 kg/m2; CON: 28.9 ± 4.4 kg/m2). Out of the 13 studies, 921,22,24−26,30−33 were conducted during adjuvant treatment (chemotherapy, radiation, and/or androgen deprivation therapy), whereas 423,27−29 were conducted after the completion of treatment.
Table 1.
Summary of included studies.
| Age (year) |
n (% female) |
BMI (kg/m2) |
Intervention |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | RT | CON | RT | CON | RT | CON | Cancer type | Patient or survivor | Treatment type | Treatment stage | Duration (week) | Frequency (per week) | Volume | Session length (min) | Intensity |
| Alberga et al. (2012)21 | 67.1 ± 6.9 | 65.4 ± 7.6 | 40 (0) | 41 (0) | 28.1 ± 3.5 | 29.0 ± 4.2 | Prostate | Patient | ADT | N/A | 24 | 3 | 10 × 2 × (8−12) | N/A | 60%−70% of 1-RM |
| Benton et al. (2014)23 | 68.3 ± 6.8 | N/A | 8 (100) | N/A | 27.4 ± 2.8 | N/A | Breast | Survivor | No treatment | N/A | 8 | 2 | 8 × 3 × (8−12) | N/A | 50%−80% of 1-RM |
| Nilsen et al. (2015)24 | 66.0 ± 6.6 | 66.0 ± 5.0 | 28 (0) | 30 (0) | 29.1 ± 3.9 | 28.4 ± 3.4 | Prostate | Patient | ADT, Radiotherapy | N/A | 16 | 3 | 9 × (1−3) × (6−10) | N/A | 80%−100% of 10-RM, 6-RM |
| Nilsen et al. (2016)33 | 67.0 ± 7.0 | 64.0 ± 6.0 | 12 (0) | 11 (0) | 28.0 ± 2.3 | 29.8 ± 3.5 | Prostate | Patient | ADT, Radiotherapy | N/A | 16 | 3 | 9 × (1−3) × (6−10) | N/A | 80%−100% of 10-RM, 6-RM |
| Cormie et al. (2013)27 | 73.1 ± 7.5 | 71.2 ± 6.9 | 10 (0) | 10 (0) | 29.1 ± 3.1 | 28.3 ± 4.0 | Prostate | Patient | No treatment | N/A | 12 | 2 | 8 × (2−4) × (8−12) | 60 | 8−12-RM |
| Cormie et al. (2014)28 | 70.0 ± 9.8 | N/A | 20 (15) | N/A | 28.5 ± 3.5 | N/A | Breast,Prostate | Patient | No treatment | N/A | 12 | 2 | 8 × (2−4) × (8−12) | 60 | 8−12-RM |
| Rosenberger et al. (2017)26 | 65.0 ± 11.0 | 61.0 ± 6.0 | 10 (20) | 10 (20) | 25.9 ± 3.9 | 24.1 ± 2.6 | Renal, Gastrointestinal | Patient | Therapies with tyrosine kinase inhibitors | N/A | 12 | 2 | 8 × 2 × 12 | 60 | 12-RM |
| Segal et al. (2003)25 | 68.2 ± 7.9 | 67.7 ± 7.5 | 82 (0) | 73 (0) | 29.0 ± 3.5 | 28.5 ± 3.7 | Prostate | Patient | ADT | 2−4 | 12 | 3 | 9 × 2 × (8−12) | N/A | 60%−70% of 1-RM |
| Segal et al. (2009)22 | 66.4 ± 7.6 | 65.3 ± 7.6 | 40 (0) | 41 (0) | 28.1 ± 3.5 | 29.0 ± 4.2 | Prostate | Patient | ADT | 2−4 | 24 | 3 | 10 × 2 × (8−12) | N/A | 60%−70% of 1-RM |
| Simonavice et al. (2017)29 | 64.0 ± 7.0 | N/A | 27 (100) | N/A | 27.7 ± 5.5 | N/A | Breast | Survivor | No treatment | 0−3 | 24 | 2 | 10 × 2 × (8−12) | N/A | 60%−80% of 1-RM |
| Winters-Stone et al. (2011)30 | 62.3 ± 6.7 | 62.2 ± 6.7 | 52 (100) | 54 (100) | 29.5 ± 5.8 | 29.5 ± 5.6 | Breast | Survivor | Chemotherapy, Radiotherapy | 0−3 | 48 | 3 | 10 × (1−4) × (8−12) | 45–60 | 60%−70% of 1-RM |
| Winters-Stone et al. (2012)31 | 62.3 ± 6.7 | 62.2 ± 6.7 | 36 (100) | 31 (100) | 29.5 ± 5.8 | 29.5 ± 5.6 | Breast | Survivor | Chemotherapy, Radiotherapy | 0−3 | 48 | 3 | 9 × (1−3) × (8−12) | N/A | 60%−80% of 1-RM |
| Winters-Stone et al. (2015)32 | 69.9 ± 9.3 | 70.5 ± 7.8 | 29 (0) | 22 (0) | 28.4 ± 4.1 | 29.6 ± 4.8 | Prostate | Survivor | ADT, Chemotherapy, Radiotherapy | N/A | 48 | 3 | 10 × 3 × (10−12) | N/A | 60%−70% of 1-RM |
Notes: Age and BMI are presented as mean ± SD. Volume is presented as number of exercise × sets × repetitions.
Abbreviations: ADT = androgen deprivation therapy; BMI = body mass index; CON = control group; N/A = not available; RM = repetition maximum; RT = resistance training group.
3.5. Interventions
All interventions were supervised in research centers by qualified trainers or researchers using free weights or weight machines. All training targeted both upper and lower body. The mean training period was 23 weeks (minimum–maximum: 8 weeks23–48 weeks30−32). In all studies, training frequency was 2 or 3 days per week. All training consisted of 1–4 sets, 6–12 repetitions, and 8–10 exercises. The number of total sets per week ranged from 3226 to 90,32 and the number of total repetitions per week ranged from 38426 to 990.32 All trials established their intensity by percentage of 1-RM or 6–12-RM (a workload that enables the participant to complete 6–12 repetitions). Intensities ranged from moderate (50% of 1-RM23) to high (100% of 6-RM24,33). All trials except for 224,33 progressively increased their intensity over the duration of the intervention on the basis of the progression of 1-RM tested at baseline and mid-study. The majority of included studies did not provide information regarding session duration, rest periods, or the use of momentary muscular failure with verbal encouragement.
3.6. Measurements
Of all 13 cohorts, 10 trials assessed muscular strength by measuring maximum weight moved.21, 22, 23, 24,27, 28, 29,31, 32, 33 Two trials measured maximal voluntary isometric contraction26 or the number of repetitions,25 and 1 trial did not measure strength.30 Six trials assessed muscle hypertrophy by measuring lean body mass by dual X-ray absorptiometry,21,24,27−30 and 1 trial measured muscle fiber CSA by Tema Image-Analysis System.33
3.7. Muscular strength
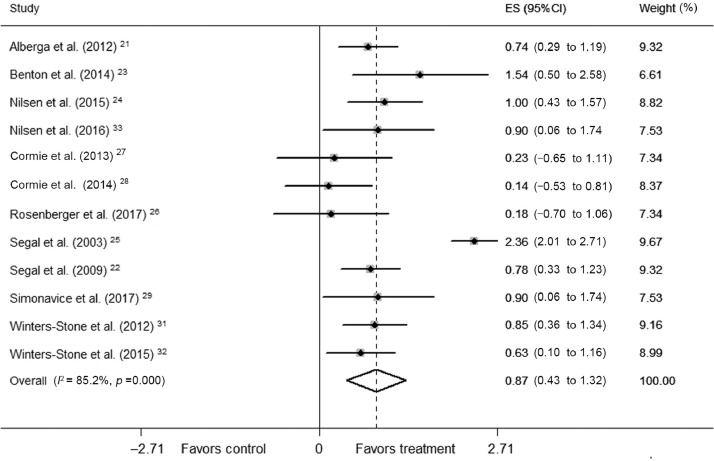
RT in 12 trials significantly increased muscular strength (mean ES = 0.87, 95%CI: 0.43–1.32, p < 0.001) (Fig. 3). The absolute increase of muscular strength was 23.61%. Univariate meta-regression showed high heterogeneity between studies (Q = 74.47, df = 11; I2 = 85.2%, p < 0.001). In subgroup analyses, there was no significant difference in effect between male and female subgroups (p = 0.65). In terms of training variables, significant difference in effect was not found between subgroups training for <24 weeks and ≥24 weeks (p = 0.69), moderate intensity (50–70% of 1-RM) and high intensity (70%–90% of 1-RM) (p = 0.75), 2 days per week and 3 days per week (p = 0.29), <60 sets per week and ≥60 sets per week (p = 0.79), and <540 repetitions per week and ≥540 repetitions per week (p = 0.37).
Fig. 3.
Forest plot of ES and 95%CIs for 12 cohorts representing muscular strength, based on the random effects meta-analysis results. 95%CI = 95% confidence interval; ES = effect size.
3.8. Muscle hypertrophy
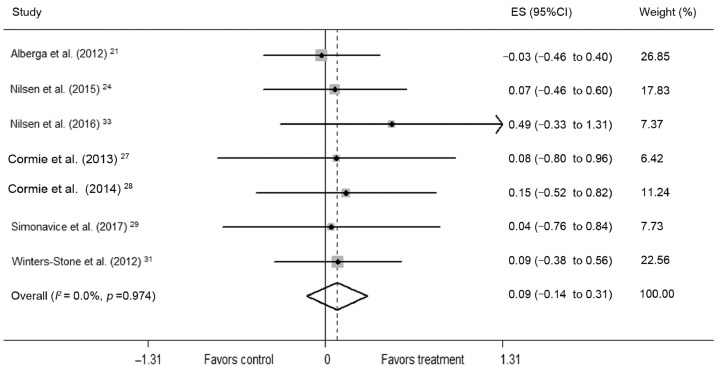
RT in 7 trials did not significantly induce muscle hypertrophy (mean ES = 0.09, 95%CI: –0.14 to 0.31, p = 0.45) (Fig. 4). The absolute increase of muscle hypertrophy was 16.25%. Univariate meta-regression did not show heterogeneity between studies (Q = 1.26, df = 6; I2 = 0.0%, p = 0.974). In subgroup analyses, there was no significant difference in effect between male and female subgroups (p = 0.96). In terms of training variables, significant difference in effect was not found between subgroups training for <24 weeks and ≥24 weeks (p = 0.55), moderate intensity (50%–70% of 1-RM) and high intensity (70%–90% of 1-RM) (p = 0.67), 2 days per week and 3 days per week (p = 0.95), <60 sets per week and ≥60 sets per week (p = 0.95), and <540 repetitions per week and ≥540 repetitions per week (p = 0.60).
Fig. 4.
Forest plot of ES and 95%CIs for 7 cohorts representing muscular hypertrophy, based on the fixed effects meta-analysis results. 95%CI = 95% confidence interval; ES = effect size.
3.9. Sensitivity analysis
Sensitivity analysis reported that by excluding any of all cohorts from the meta-analysis the estimated effects will still be within the 95%CI of the mean ES in both 2 outcomes, suggesting the results of the meta-analysis will not significantly change after the removal of any one cohort.
4. Discussion
The primary results of this meta-analysis study are that RT significantly increased muscular strength by 23.61% but insignificantly induced muscle hypertrophy by 16.25% in cancer patients of an average age of 66.4 years. An increase of 22.1 kg in lower limb muscle strength was achieved by RT. An increase of 10.7 kg in leg press is associated with a 35% decreased risk of cancer mortality in men, independent of cardiorespiratory fitness and body fat.9 Moreover, muscle loss greater than 5% is associated with a 2 times higher cancer mortality rate.10 Therefore, it is noted that RT could substantially contribute to improve health in elderly cancer patients by enhancing muscle function.
High heterogeneity was found between studies on muscular strength. However, significant differences in effect were not found between subgroups training for durations of short/middle-term (<24 weeks) and middle/long-term (≥24 weeks), at moderate intensity (50%–70% of 1-RM) and high intensity (70%–90% of 1-RM), with lower weekly frequency (2 days per week) and higher weekly frequency (3 days per week), and with lower (<60 sets or <540 repetitions per week) and higher training volume (≥60 sets or ≥540 repetitions per week). These results suggest that RT would improve muscle strength in elderly cancer patients by widely satisfying patients’ preferences, e.g., for a lower or higher training volume. In connection with training volume, 5 of 7 subgroups training 3 days per week performed at a higher volume, while all of 5 subgroups training 2 days per weeks performed at a lower volume. These results seem to be natural as weekly training volume increases in proportion to weekly frequency. However, training intensity is another major factor that is inversely associated with training volume in general. In this study, 4 of 5 subgroups training with higher intensity performed at a lower training volume, suggesting that training volume does not depend exclusively on weekly frequency. Based on subgroup analyses, no training variables lead to a significant difference in effect. In other words, we found that muscular strength in elderly cancer patients could be significantly increased through RT applied at moderate intensity for relatively short periods with a lower training volume and weekly frequency. These results are partially similar to a previous meta-analysis study which reported that weekly frequency and volume of RT have no statistically significant association with strength gains in middle-aged cancer patients.14 In this study, however, there is a bit of discrepancy between the subgroups sorted by training frequency with regard to the number of studies. Moreover, other essential elements, such as session duration, rest periods, and the use of momentary muscular failure, were not reported in most included studies. Thus, additional studies are needed to investigate these variables.
In subgroup analyses based on demographic variables, most cancer patients were in various cancer treatments (e.g., chemotherapy, radiation, and androgen deprivation therapy). Thus, further analysis based on the types of treatment and the stage of cancer could not be carried out. A recent meta-analysis study reported that RT increases muscle strength independent of treatment type.15 Since most cancer patients in this study had a normal body weight (BMI < 30 kg/m2), it is possible that different training strategies could be required for obese cancer patients. As the patients in this study were mostly male, it is difficult to determine a sex difference in effects of RT on outcomes. Future research is warranted to investigate these variables.
In terms of muscle hypertrophy, there are some possible reasons for no significant change after RT. First, muscular hypertrophic responses to RT may be decreased according to an increase in training periods due to physiological adaptations. Although significant difference in effect was not found between subgroups training for <24 weeks and ≥24 weeks (p = 0.55), 4 included studies training for <24 weeks reported that RT significantly increases lean body mass24,27,28 or muscle fiber CSA,33 whereas 3 studies training for ≥24 weeks reported that RT does not result in a significant effect on muscle hypertrophy.21,29,30 In particular, resistance was progressively increased for the middle/long-term training. Other training elements may have to be adjusted throughout the intervention, such as exercise type, volume, repetition speed, and momentary muscular failure as the point of set termination in order to promote continuous muscle hypertrophy. Future research is warranted to ascertain the influence of these diverse variables on muscle hypertrophy. Another possible reason for the insignificant muscle hypertrophy is that additional dietary interventions, such as a protein supplement or a modified higher protein diet, were not incorporated in all studies, which could result in diminished muscle protein synthesis. According to a recent meta-analysis,34 the use of protein supplements alongside RT induces muscle hypertrophy as well as strength in elderly populations more than RT without protein supplements. To the best of our knowledge, there have been few studies investigating the effects of protein consumption on muscle hypertrophy following RT. Thus, further studies are needed for identifying them.
There are some limitations to this study. First, the number of included studies might not be adequate. Some studies were excluded to enhance homogeneity. Also, only studies published in English were retrieved, which could increase the risk of bias; however, significant publication bias was not found in either outcome. Second, 12 of the 13 studies were conducted on breast and/or prostate cancer patients, thus limiting the generalizability of this review to all cancer patients. Lastly, 3 of the included studies did not involve CON.23,28,29 However, differences in ESs are insignificant between randomized and nonrandomized trials.35
Despite these limitations, this meta-analysis study has significant strengths. This is the first study to investigate the effects of RT on both muscular strength and hypertrophy in elderly cancer patients. To enhance the validity of the research, this review did exclude studies that targeted cancer patients aged <60 years and/or those that conducted RT combined with aerobic training or RT for <6 weeks. It has been suggested that muscle hypertrophy usually occurs after 6 weeks of training.36 Furthermore, this review determined that gains in muscle strength can be significantly induced by RT and that no training variables lead to significant differences in effect. Thus, this study reveals the importance of RT on muscle function in elderly cancer patients and contributes to establishing a specified RT strategy for the patients.
This review suggests that an intensity of moderate (50%–70% of 1-RM), a frequency of 3 days per week, and a duration of 8–12 weeks should be considered a priority in RT programs for greater effect on muscle strength gains in elderly cancer patients. For detailed training variables, a volume of 10–12 repetitions, 3 sets, and 10 large muscle group exercises can be an additional recommendation. To promote muscle hypertrophy, it is necessary to adjust various training elements during middle/long-term training (≥24 weeks). For greater effect with a minimum of exercise-induced injuries or adverse events in the elderly patients, resistance should be gradually increased under the direction of practitioners throughout the training period. The RT guidelines recommended in this review are not much different from the recommendations for cancer survivors given by the American College of Sports Medicine (ACSM) this is to be expected as the majority of included studies followed the ACSM guidelines.37 Importantly, the RT guidelines in this review should be differently applied to patients with consideration of the physical condition of each individual. Future research should investigate various training elements beyond the traditional ACSM guidelines. For example, low-load RT can be carried out to momentary muscular failure with short rest periods between sets because high-intensity training can be defined as not only heavy loads lifted but also light loads with high repetitions and short rest periods. Moreover, it is well-known that chemotherapy can reduce bone density and increase frailty in elderly cancer patients. As chemotherapy is also associated with muscle loss,10 it would be helpful to understand whether previous RT experience, prior to chemotherapy, influences the response to RT during or following chemotherapy. In summary, researchers should seek to determine those various factors related to RT in order to establish further optimization of exercise guidelines.
5. Conclusion
This systematic review and meta-analysis found that RT significantly increased muscular strength but induced insignificant muscle hypertrophy in elderly cancer patients. Subgroup analyses determined that no training variables lead to significant differences in effect, which suggests that RT would improve muscle strength in elderly cancer patients through an appropriate RT strategy flexibly applied depending on the patients’ preferences. It is expected that traditional RT guidelines would induce marginal muscle hypertrophy in the elderly patients. In future investigations, researchers may wish to examine various training components in detail to establish further optimization of exercise guidelines.
Acknowledgments
Acknowledgment
The author thanks all included study authors who provided additional information.
Competing interests
The author declares that he has no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.Bozzetti F. Evidence-based nutritional support of the elderly cancer patient. Nutrition. 2015;31:585–586. doi: 10.1016/j.nut.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Viganò A, Morais JA. The elderly patient with cancer: A holistic view. Nutrition. 2015;31:587–589. doi: 10.1016/j.nut.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Evans WJ, Morley JE, Argilés J, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50:147–150. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 6.Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13:708–712. doi: 10.1007/s12603-009-0201-z. [DOI] [PubMed] [Google Scholar]
- 7.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morishita S, Tsubaki A, Fu JB. In: Understanding cachexia, sarcopenia, and physical exercise in patients with cancer. Dionyssiotis Y, editor. IN TECH; Rijeka: 2017. Physical therapy in patients with cancer. Frailty and sarcopenia onset, development and clinical challenges; pp. 123–132. [Google Scholar]
- 9.Ruiz JR, Sui X, Lobelo F, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev. 2009;18:1468–1476. doi: 10.1158/1055-9965.EPI-08-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamoto Y, Baba Y, Sakamoto Y, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borde R, Hortobágyi T, Granacher U. Dose–response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med. 2015;45:1693–1720. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: Changes in evoked V-wave and H-reflex responses. J Appl Physiol (1985) 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 13.Al-Majid S, Waters H. The biological mechanisms of cancer-related skeletal muscle wasting: The role of progressive resistance exercise. Biol Res Nurs. 2008;10:7–20. doi: 10.1177/1099800408317345. [DOI] [PubMed] [Google Scholar]
- 14.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: A meta-analysis. Med Sci Sports Exerc. 2013;45:2080–2090. doi: 10.1249/MSS.0b013e31829a3b63. [DOI] [PubMed] [Google Scholar]
- 15.Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: A meta-analysis. J Cancer Surviv. 2017;11:339–349. doi: 10.1007/s11764-016-0592-x. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 18.Higgin JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. 2nd ed. Lawrence Erlbaum Associates; New York, NY: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberga AS, Segal RJ, Reid RD, et al. Age and androgen-deprivation therapy on exercise outcomes in men with prostate cancer. Support Care Cancer. 2012;20:971–981. doi: 10.1007/s00520-011-1169-x. [DOI] [PubMed] [Google Scholar]
- 22.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 23.Benton MJ, Schlairet MC, Gibson DR. Change in quality of life among breast cancer survivors after resistance training: Is there an effect of age? J Aging Phys Act. 2014;22:178–185. doi: 10.1123/japa.2012-0227. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen TS, Raastad T, Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805–1813. doi: 10.3109/0284186X.2015.1037008. [DOI] [PubMed] [Google Scholar]
- 25.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberger F, Wiskemann J, Vallet S, et al. Resistance training as supportive measure in advanced cancer patients undergoing TKI therapy—A controlled feasibility trial. Support Care Cancer. 2017;25:3655–3664. doi: 10.1007/s00520-017-3788-3. [DOI] [PubMed] [Google Scholar]
- 27.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328–335. doi: 10.1038/pcan.2013.22. [DOI] [PubMed] [Google Scholar]
- 28.Cormie P, Galvão DA, Spry N, Joseph D, Taaffe DR, Newton RU. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: Longitudinal results of a pilot study. Support Care Cancer. 2014;22:1537–1548. doi: 10.1007/s00520-013-2103-1. [DOI] [PubMed] [Google Scholar]
- 29.Simonavice E, Kim JS, Panton L. Effects of resistance exercise in women with or at risk for breast cancer-related lymphedema. Support Care Cancer. 2017;25:9–15. doi: 10.1007/s00520-016-3374-0. [DOI] [PubMed] [Google Scholar]
- 30.Winters-Stone KM, Dobek J, Nail L, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: A randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: A randomized controlled trial. J Cancer Surviv. 2012;6:189–199. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winters-Stone KM, Dobek JC, Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: Evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:7–14. doi: 10.1016/j.apmr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsen TS, Thorsen L, Fosså SD, et al. Effects of strength training on muscle cellular outcomes in prostate cancer patients on androgen deprivation therapy. Scand J Med Sci Sports. 2016;26:1026–1035. doi: 10.1111/sms.12543. [DOI] [PubMed] [Google Scholar]
- 34.Cermak NM, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 35.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 36.Souza EO, Ugrinowitsch C, Tricoli V, et al. Early adaptations to six weeks of non-periodized and periodized strength training regimens in recreational males. J Sports Sci Med. 2014;13:604–609. [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.