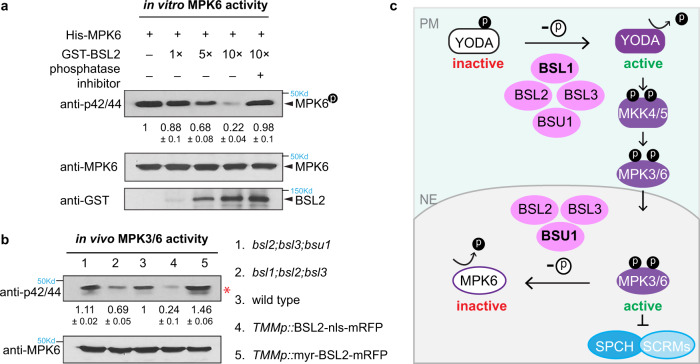
Fig. 6. Negative regulation of BSL phosphatases on MAPKs in vitro and in vivo.
a Kinase activity of the recombinant MPK6 protein in the presence or absence of BSL2 was examined in vitro. MPK6 and BSL2 were fused histidine-tag (His) and glutathione S-transferase (GST), respectively. phosphorylated MPK6 was detected by immunoblot using anti-phospho-p42/p44 antibody. Protein levels of His-MPK6 or GST-BSL2 were examined using anti-MPK6 or anti-GST antibody, respectively. Numbers indicate relative amount of phosphorylated MPK6 protein of three biological replicates. Data are presented as mean ± SD. b Phosphorylated MPK3/6 (activity) levels in 5-dpg Arabidopsis seedlings were detected by anti-phospho-p42/p44 (top). Total proteins were extracted, and immunoblotting assays were performed using anti-phospho-p42/p44 (top) and anti-MPK6 (bottom). Numbers are mean ± SD, intensity quantification of phosphorylated MPK6 bands (red asterisk). n = three biological replicates. a, b Representative results of assays performed three times. c A working model: A BSL phosphatases-based signaling dichotomy, through spatial compartmentalization of the regulation on distinct components of the linear YODA MAPK pathway, controls stomatal development in Arabidopsis. At the cell cortex close to the PM, BSL1 is a predominant regulator, together with the other three BSL phosphatases, activating the MAPKKK YODA to promote MAPK signaling. Activated MPK3/6 molecules phosphorylate the key stomatal fate transcription factors, SPCH and ICE1/SCRMs, for degradation, thereby suppressing stomatal production. In the nucleus, BSU1 plays a primary role, together with BSL2 and BSL3, deactivating MPK3/6, resulting in stabilized SPCH and ICE1/SCRMs, thereby promoting stomatal production.