Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, patients with acute respiratory distress syndrome (ARDS) due to SARS-CoV-2 showed a profoundly altered immune system and received immune-modulating therapeutic interventions. This enhanced the susceptibility for fungal superinfections [1, 2]. With the first reports of COVID-19-associated pulmonary aspergillosis (CAPA) the 2020 European Confederation of Medical Mycology (ECMM)/International Society for Human and Animal Mycology (ISHAM) consensus criteria were proposed [3, 4] and Aspergillus tracheobronchitis (ATB) was distinguished as a sub-entity in CAPA [4–6]. During bronchoscopy, ATB presents as ulcerations, pseudomembranes, plaques and eschars, possibly combined with tracheal stenosis [5]. Facing the risk of transmission and SARS-CoV-2 infection of examiners during bronchoscopy, blind suctioning of upper airway samples has been implemented with tracheal aspirates (TA) and non-bronchoscopic lavages. These techniques preclude inspection of the airways, so that ATB cannot be diagnosed beyond the level of suspicion. To study ATB in CAPA patients, we performed a retrospective, single-centre cohort study.
Short abstract
Comprehensive work-up is needed for COVID-19 ARDS patients, especially when suspecting invasive fungal infections. Aspergillus tracheobronchitis has a substantial prevalence in patients with CAPA accounting for an overall mortality of 75% in this study. https://bit.ly/3uF3FZU
To the Editor:
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, patients with acute respiratory distress syndrome (ARDS) due to SARS-CoV-2 showed a profoundly altered immune system and received immune-modulating therapeutic interventions. This enhanced the susceptibility for fungal superinfections [1, 2]. With the first reports of COVID-19-associated pulmonary aspergillosis (CAPA) the 2020 European Confederation of Medical Mycology (ECMM)/International Society for Human and Animal Mycology (ISHAM) consensus criteria were proposed [3, 4] and Aspergillus tracheobronchitis (ATB) was distinguished as a sub-entity in CAPA [4–6]. During bronchoscopy, ATB presents as ulcerations, pseudomembranes, plaques and eschars, possibly combined with tracheal stenosis [5]. Facing the risk of transmission and SARS-CoV-2 infection of examiners during bronchoscopy, blind suctioning of upper airway samples has been implemented with tracheal aspirates (TA) and non-bronchoscopic lavages. These techniques preclude inspection of the airways, so that ATB cannot be diagnosed beyond the level of suspicion. To study ATB in CAPA patients, we performed a retrospective, single-centre cohort study.
We analysed data from patients treated for COVID-19-associated ARDS at the 14-bed internal medicine intensive care unit (ICU) at our hospital. The study period was March 2020 to February 2021. Patients were included in the FungiScope registry (https://www.clinicaltrials.gov; NCT01731353; EC approval: 05-102) [7]. Data on patient demographic characteristics were collected by chart review. Non-bronchoscopy data has in part been analysed and published previously [2, 8]. At the pandemic's beginning, we implemented a screening for CAPA (biweekly analysis of TA for Aspergillus-PCR, galactomannan and culture combined with serum galactomannan). In case of positive results, bronchoscopy and bronchoalveolar lavage (BAL) followed. Aspergillus 28S rDNA-Realtime PCR was established as in-house assay. Species identification was performed by artus Aspergillus diff. RG PCR kit (Qiagen, Hilden, Germany). For galactomannan testing from serum, BAL fluid or TA, Platelia Aspergillus antigen ELISA (Bio-Rad Laboratories Inc., Hercules, USA) was used. In culture-positive cases the VIPcheck (Mediaproducts BV, Groningen, the Netherlands) was used to rule out resistance [9]. To define CAPA, the 2020 ECMM/ISHAM consensus criteria were applied [4]. To analyse the demographic and clinical characteristics, we describe categorical variables using frequencies and percentages and continuous variables using medians, interquartile ranges (IQRs) and range. SPSS v25.0 was employed for statistical analyses (SPSS, IBM Corp., Chicago, USA). Figure 1b–e are included for illustration purposes by courtesy of S. von Stillfried and P. Boor. The clinical autopsy was performed at the Institute of Pathology at the RWTH Aachen, Germany following approval by the next of kin (EC approval: EK 304/20, EK 119/20, and EK 092/20).
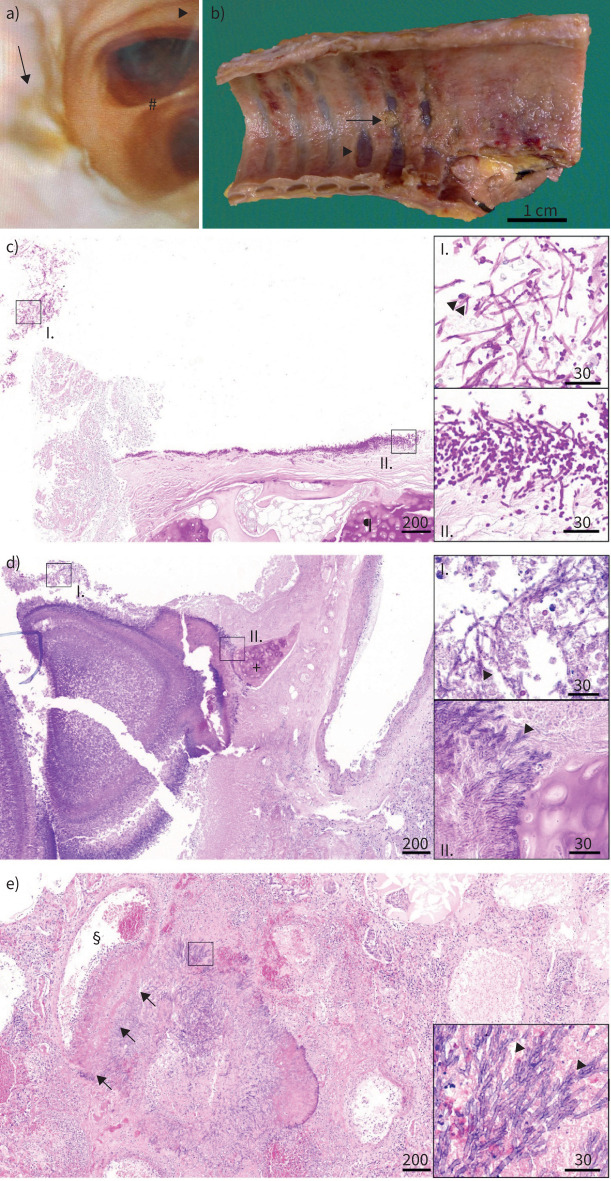
FIGURE 1.
Aspergillus tracheobronchitis. a) Bronchoscopy image of a patient with Aspergillus tracheobronchitis with tracheal ulceration and plaque formation (arrow). Bird's-eye view from the middle section of the trachea down into the primary bronchi separated by the carina (#). Notice the mucosa as it should be at the carina (#) and at the surrounding of the arrowhead, which points to the ventral part of the trachea. b) Macroscopic image of tracheal luminal surface at autopsy from a patient with invasive Aspergillus tracheobronchitis and invasive pulmonary aspergillosis. Note erosion and ulceration of tracheal mucosa with exposition of tracheal cartilage (arrowhead), partially covered by detached cellular detritus (arrow, histology shown in panel c). c) Images from tracheal and lung autopsy tissue from a coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS) patient with Aspergillus tracheobronchitis and invasive pulmonary aspergillosis. Fungal invasion of the tracheal mucosa, fungal hyphae with 45° branching, 2–4 µm in diameter, consistent with Aspergillus spp. ¶: tracheal cartilage, PAS 4×, scale bar 200 µm; insert I: detached cellular detritus with fungal hyphae (arrowheads), PAS 40×, scale bar 30 µm; insert II: tracheal luminal surface with invasive growth of fungal hyphae with 45° branching, 2–4 µm in diameter, consistent with Aspergillus spp., PAS 40×, scale bar 30 µm. Due to ossification of tracheal cartilage, tissue was decalcified in formic acid overnight. d) Aspergillus invasion of the bronchial mucosa, fungal hyphae with 45° branching, 2–4 µm in diameter, typical for Aspergillus spp. +: bronchial cartilage, PAS 4×, scale bar 200 µm; insert I: detached cellular detritus with fungal hyphae (arrowheads), PAS 40×, scale bar 30 µm; insert II: bronchial wall with invasive growth of fungal hyphae (arrowheads) reaching bronchial cartilage, PAS 40×, scale bar 30 µm. e) Pulmonary vascular invasion of Aspergillus, fungal hyphae with 45° branching, 2–4 µm in diameter, typical for Aspergillus spp. §: vascular lumen, arrows: vascular wall with fungal invasion, HE 4×, scale bar 200 µm; insert: fungal hyphae with 45° branching, typical for Aspergillus spp. (arrowheads), HE 40×, scale bar 30 µm. b–e) Courtesy of S. von Stillfried and P. Boor (both Institute of Pathology, RWTH Aachen University Hospital, Aachen, Germany).
A total of 69 COVID-19 patients were admitted to our ICU during the 1-year observation period. There were a higher prevalence of males (COVID-19: n=40, 76.9%; CAPA: n=12, 70.6%) and higher proportion of patients with Caucasian ethnic background in both cohorts, respectively (COVID-19: n=32, 61.5%; CAPA: n=11, 64.7%). Patients diagnosed with CAPA had longer stays on the ICU, with a median of 20 days (IQR 14–22, range 4–255 days). The majority of patients received prone positioning cycles due to ARDS during their ICU stays (COVID-19: 86.5%, CAPA: 88.2%) and a total of 11 patients (COVID-19: n=9, 17.3%; CAPA: n=2, 11.8%) underwent extracorporeal membrane oxygenation. COVID-19 treatment approaches were mainly dexamethasone (COVID-19: n=39, 75%; CAPA: n=10, 58.8%) and remdesivir (COVID-19: n=13, 25%; CAPA: n=6, 35.3%). A total of 66 of 69 (95.7%) patients received antibiotic therapy during the stay on ICU. Of those, 53 patients were treated with more than one antibiotic. Median treatment duration was 14 days (IQR 9.25–17.75, range 2–49 days) for the total cohort. In CAPA patients, median duration was 15.5 days (IQR 12.25–24, range 5–49 days) versus non-CAPA patients 14 days (IQR 8.75–17, range 2–48 days).A proportion of 13.5% of non-CAPA patients (8/52) showed an immunocompromising underlying disease, versus 11.8% (2/17) in the CAPA cohort. Upon admission, a median arterial oxygen tension (PaO2)/inspired oxygen fraction (FIO2) index of 150.5 (IQR 108–205, range 37.6–453) was documented for non-CAPA patients in comparison to 97.3 (IQR 81.4–173, range 42.9–314) for CAPA patients. Bronchoscopy was performed in 40 (76.9%) of the non-CAPA and all (n=17) CAPA patients. White-coloured plaques were reported in 41.2% of CAPA cases (n=7). Pseudomembranes were reported in 41.2% CAPA patients and the clinical diagnosis of tracheobronchitis was established in 47.1% of CAPA patients (figure 1a).
We observed 8/17 (47.1%) CAPA patients with clinical diagnosis of ATB during bronchoscopy. Non-ATB CAPA patients had longer ICU stays with a median of 21 days (IQR 19–28, range 4–255 days), in contrast to CAPA ATB patients with 14.5 days in the ICU (IQR 11–21, range 6–64 days). This is mirrored in a higher day-30 mortality in ATB patients (ATB day-30 mortality: n=5, 62.5%; overall mortality: n=6, 75%; versus non-ATB day-30 mortality: n=2, 22.2%; overall mortality: n=3, 33.3%; overall mortality in all CAPA patients: 52.9%). Bronchoscopy revealed tracheal plaques in all ATB patients, with seven (87.6%) of white and one (12.5%) of dark colour. Additionally, pseudomembranes (n=7), thrombi (n=4) and a vulnerable or bloody trachea (n=7) were reported in 87.5%, 50% and 87.5%, respectively. In 6/8 (75%) tracheobronchitis patients, BAL samples were tested positive for galactomannan-antigen index of >0.5. Seven cultures (87.5%), as well as eight PCR tests from patients with tracheobronchitis were positive for Aspergillus (n=8, 100%). Conversely, half of the non-tracheobronchitis group yielded a positive culture (n=5, 55.6%) or PCR (n=5, 55.6%) result. The dominant species identified was Aspergillus fumigatus (tracheobronchitis: n=7, 87.5%; non-tracheobronchitis: n=3, 33.3%). Serum galactomannan showed significant limitations since only one ATB patient and two without tracheobronchitis tested positive. In contrast, 6/8 ATB patients had positive BAL galactomannan and all had a positive culture (no azole resistance detected). Applying the ECMM/ISHAM definitions, all CAPA patients had probable disease. Antifungal drugs used were voriconazole (ATB: n=6, 75%; non-tracheobronchitits n=6, 66.7%) and isavuconazole (ATB n=4, 50%; non-tracheobronchitis: isavuconazole n=2, 22.2%).
During the first wave of the COVID-19 pandemic, bronchoscopy has played a limited role. By lack of tracheal examination, local ulcerations, pseudomembranes and lesions are not diagnosed (figure 1). Samples obtained by TA or non-bronchoscopic lavages show reduced diagnostic quality. Our cohort presents insights about ATB in CAPA patients. Our results suggest that identifying the presence of plaques and ulceration are crucial for diagnosis (figure 1) [6]. The use of computed tomography scans can hardly differentiate ATB from non-ATB patients. In particular, Aspergillus-PCR and culture support ATB diagnosis. On the contrary serum galactomannan showed low diagnostic value, possibly due to its lack of accuracy in non-haematological patients [10]. Nevertheless, identification through biomarkers may be limited in their diagnostic accuracy due to CAPA stage specificity, so that in case of persistence or progression of tracheobronchitis or space-consuming lesions, a sampling by brush or even biopsy should be considered [6]. The day-30 mortality as well as the overall mortality rate of patients with ATB was significantly higher, emphasising the importance of early diagnosis and targeted treatment.
This single-centre study has several limitations. The data reflect a real-life scenario of critically ill COVID-19 patients, that did not undergo a pre-defined bronchoscopy protocol. The indication for bronchoscopy was adjusted to the clinical and respiratory status of the patient, triggered by positive results from tracheal aspirates and persisting fever or high volume of mucous, blood or other fluids. Tracheobronchitis in our cohort was a clinical and visual diagnosis that has been combined with the respective microbiological results taken during the procedure and not by biopsy to avoid severe injury and subsequent deterioration.
This study reveals the importance of predefined diagnostic strategies, such as indications for bronchoscopy, to identify ATB patients. ATB produces tracheal plaques, pseudomembranes and increased tracheal bleeding and is observed in a very ill patient subpopulation (PaO2/FIO2 index median 84.6). The combination of severely impaired respiratory function and aspergillosis leads to higher mortality. Key challenges for future research comprise identifying predisposing factors, also with regard to immunosuppressive treatments, such as IL-6, JAK and IL-1 inhibitors, and strategies to reduce CAPA prevalence. Prophylaxis and treatment of CAPA and ATB should be evaluated in prospective trials; especially the use of inhaled antimycotics could play a major role in patients with ATB.
Shareable PDF
Footnotes
Author contributions: P. Koehler conceived the study idea, recruited patients, collected, analysed and interpreted data and wrote the first manuscript draft. J. Salmanton-García and F. Pult contributed to study design, data interpretation and revision of the first manuscript draft. S. von Stillfried and P. Boor provided figure 1b–e. M. Kochanek, F. Fuchs, B. Böll, J. Garcia Borrega, D.A. Eichenauer, A. Shimabukuro-Vornhagen and O.A. Cornely recruited patients and collected and interpreted data. All authors contributed to manuscript writing and review of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest: P. Koehler reports grants or contracts from German Federal Ministry of Research and Education and the State of North Rhine-Westphalia; consulting fees from Ambu GmbH, Gilead Sciences, Noxxon N.V. and Pfizer Pharma; honoraria for lectures from Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, BioRad Laboratories Inc., European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, medupdate GmbH, MedMedia, MSD Sharp & Dohme GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica SC and University Hospital and LMU Munich; participation on an advisory board from Ambu GmbH, Gilead Sciences and Pfizer Pharma; a pending patent currently reviewed at the German patent and trade mark office (official file number DE 10 2021 113 007.7); other non-financial interests from Elsevier, Wiley and Taylor & Francis online outside the submitted work.
Conflict of interest: S. von Stillfried has nothing to disclose.
Conflict of interest: J. Garcia Borrega reports scientific grants and travel expenses from Kite/Gilead outside the submitted work.
Conflict of interest: F. Fuchs has a clinician scientist position supported by the dean's office, medical faculty, University of Cologne.
Conflict of interest: J. Salmanton-García has nothing to disclose.
Conflict of interest: F. Pult has nothing to disclose.
Conflict of interest: B. Böll reports honoraria, travel expenses and advisory role from/for Astellas, Celgene, Johnson & Johnson, Kite/Gilead, MSD, Novartis, Pfizer and Takeda, and financing of scientific research by Astellas, Celgene, Kite/Gilead, MSD and Takeda outside the submitted work.
Conflict of interest: D.A. Eichenauer received honoraria from Sanofi and Takeda outside the submitted work.
Conflict of interest: A. Shimabukuro-Vornhagen reports travel grants from Gilead Sciences outside the submitted work.
Conflict of interest: O. Kurzai reports payment or honoraria for lectures, presentations or speakers' bureaus by Gilead and Pfizer, and receipt of equipment, materials, drugs, medical writing, gifts or other services by Pfizer, MSD, Basilea, Gilead, Virotech and Wako Fujifilm outside the submitted work.
Conflict of interest: P. Boor has nothing to disclose.
Conflict of interest: M. Kochanek reports payment or honoraria for lectures, presentations or speakers' bureaus by Gilead, MSD and Pfizer outside the submitted work.
Conflict of interest: O.A. Cornely reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan and Pfizer; payment for expert testimony from Cidara; participation on a data safety monitoring board or advisory board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI and Shionogi; a pending patent currently reviewed at the German patent and trade mark office (official file number DE 10 2021 113 007.7); other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley outside the submitted work.
Support statement: This work was supported by the German Registry of COVID-19 Autopsies (www.DeRegCOVID.ukaachen.de), funded by the Federal Ministry of Health (ZMVI1-2520COR201) and by the Federal Ministry of Education and Research within the framework of the Network of University Medicine (DEFEAT PANDEMIcs, 01KX2021). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Koehler P, Cornely OA, Bottiger BW, et al. . COVID-19 associated pulmonary aspergillosis. Mycoses 2020; 63: 528–534. doi: 10.1111/myc.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prattes J, Wauters J, Giacobbe DR, et al. . Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect 2021; in press [ 10.1016/j.cmi.2021.08.014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij PE, Gangneux JP, Bassetti M, et al. . Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020; 1: e53–e55. doi: 10.1016/S2666-5247(20)30027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koehler P, Bassetti M, Chakrabarti A, et al. . Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21: e149–e162. doi: 10.1016/S1473-3099(20)30847-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koehler P, Bassetti M, Kochanek M, et al. . Intensive care management of influenza-associated pulmonary aspergillosis. Clin Microbiol Infect 2019; 25: 1501–1509. doi: 10.1016/j.cmi.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 6.van de Veerdonk FL, Brüggemann RJM, Vos S, et al. . COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med 2021; 9: 795–802. doi: 10.1016/S2213-2600(21)00138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidel D, Duran Graeff LA, Vehreschild M, et al. . FungiScopeTM-Global Emerging Fungal Infection Registry. Mycoses 2017; 60: 508–516. doi: 10.1111/myc.12631 [DOI] [PubMed] [Google Scholar]
- 8.Prattes J, Wauters J, Giacobbe DR, et al. . Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: results from a European confederation of medical mycology registry. Intensive Care Med 2021; 47: 1158–1160. doi: 10.1007/s00134-021-06471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buil JB, van der Lee HAL, Rijs A, et al. . Single-center evaluation of an agar-based screening for azole resistance in Aspergillus fumigatus by using VIPcheck. Antimicrob Agents Chemother 2017; 61: e01250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ku NS, Han SH, Choi JY, et al. . Diagnostic value of the serum galactomannan assay for invasive aspergillosis: it is less useful in non-haematological patients. Scand J Infect Dis 2012; 44: 600–604. doi: 10.3109/00365548.2012.657672 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03142-2021.Shareable (836.5KB, pdf)