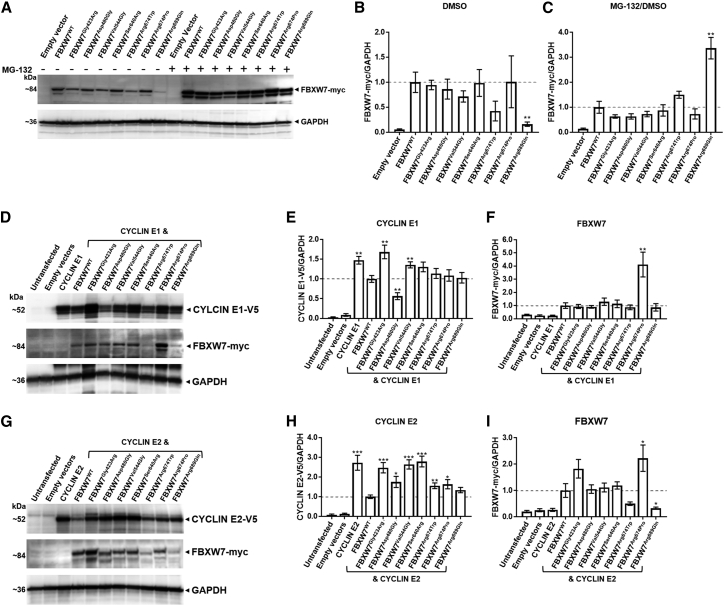
Figure 3.
Disease-associated variants impair the ability of FBXW7 to degrade substrates CYCLIN E1 and CYCLIN E2
(A) The majority of disease-associated FBXW7 variants do not impact steady-state protein amounts. Representative immunoblots of wild-type or mutant FBXW7 with and without inhibition of the ubiquitin proteasome system are shown. HEK293T cells exogenously expressing wild-type FBXW7 or mutant FBXW7 with a C-terminal Myc tag for 32 h were treated with 5 μM MG-132 for 16 h (four independent replicates).
(B) Quantification of FBXW7:GAPDH from DMSO-treated samples of mutant FBXW7 protein in (A) relative to FBXW7wild type; statistical support for altered steady-state amounts of the mutant FBXW7 protein was only evident for FBXW7Arg689Gln (p = 0.007).
(C) Quantification of FBXW7:GAPDH in MG-132-treated cells and versus their DMSO-treated counterpart in (A), demonstrating the change in steady-state mutant FBXW7 protein relative to FBXW7wild type protein after UPS inhibition; statistical support for altered steady-state protein amount was only evident for FBXW7Arg689Gln (p = 0.003).
(D) Certain FBXW7 mutant proteins demonstrate impaired CYCLIN E1 substrate degradation. Representative immunoblots of wild-type or mutant FBXW7 co-expressed with the substrate CYCLIN E1 are shown. Whole-cell lysates extracted from HEK293T cells that exogenously expressed wild-type FBXW7 or mutant FBXW7 with a C-terminal Myc tag and CYCLIN E1 with a C-terminal V5 tag for 48 h are shown (nine independent replicates).
(E) Quantification of CYCLIN E1:GAPDH in (D) for samples expressing mutant FBXW7 versus FBXW7wild type protein; statistical support for altered steady-state protein amount of CYCLIN E1 was evident for FBXW7Gly423Arg (p = 0.002), FBXW7Asp480Gly (p = 0.002), and FBXW7Val544Gly (p = 0.007).
(F) Quantification of FBXW7:GAPDH in (D) for FBXW7 mutant proteins versus FBXW7wild type protein when cells were co-transfected with CYCLIN E1. Statistical support for altered steady-state protein amount was evident only for FBXW7Arg674Pro (p = 0.005).
(G) The majority of FBXW7 mutant proteins demonstrate impaired CYCLIN E2 substrate degradation. Representative immunoblots of wild-type or mutant FBXW7 co-expressed with the substrate CYCLIN E2 are shown. Whole-cell lysates extracted from HEK293T cells that exogenously expressed wild-type FBXW7 or mutant FBXW7 with a C-terminal Myc tag and CYCLIN E2 with a C-terminal V5 tag for 48 h are shown (ten independent replicates).
(H) Quantification of CYCLIN E2:GAPDH in (G) for samples expressing FBXW7 mutant protein versus FBXW7wild type protein; statistical support for altered steady-state protein amount of CYCLIN E2 was evident for FBXW7Gly423Arg (p = 0.00005), FBXW7Asp480Gly (p = 0.02), FBXW7Val544Gly (p = 0.000005), FBXW7Ser640Arg (p = 0.000007), FBXW7Arg674Trp (p = 0.005), and FBXW7Arg674Pro (p = 0.02).
(I) Quantification of FBXW7:GAPDH in (G) for FBXW7 mutant proteins versus FBXW7wild type protein when cells were co-transfected with CYCLIN E2; statistical support for altered steady-state protein amount was evident for FBXW7Arg674Pro (p = 0.04) and FBXW7Arg674Pro (p = 0.02). All graphs present mean ± SEM. Student’s t test: ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001.