Figure 4.
Knockdown of the FBXW7 Drosophila ortholog ago, specifically in neurons, can lead to deficits in habituation learning deficits and more severe neuronal dysfunction
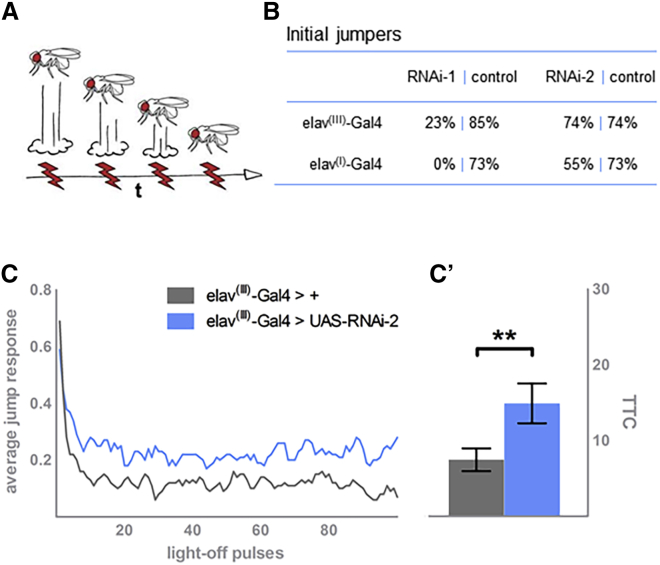
(A) Simplified scheme of the habituation assay, used for assessing the stimulus-induced escape response of individual flies upon repeated exposure. In controls, as depicted, the initial high jump response gradually wanes. Of note, in reality, the amplitude of jumps does not wane, but the frequency decreases in the tested population.
B) Knockdown of ago with RNAi-1 and either elav(I)-Gal4 or elav(III)-Gal4 severely impairs jumping. Knockdown of ago with RNAi-2 driven by either driver is less detrimental, allowing assessment of habituation learning.
(C) Neuronal knockdown of ago by elav(III)-Gal4 and RNAi-2 reduces the ability of flies to habituate to the stimulus (in blue); in ccontrast to their genetic-background controls (in gray), they keep jumping with increased frequency throughout the course of the experiment.
(D) Quantification of habituation according to mean trials to no-jump criterion (mTTC). Precise genotypes tested in (C) and (D): w/Y; 2xGMR-wIR/+; elav-Gal4(III), UAS-Dicer-2/ UAS-RNAi-2 (in blue; n = 71, mTTC = 14.91, p = 0.0015). Genetic background control w/Y; 2xGMR-wIR/+; elav-Gal4(III), UAS-Dicer-2/+ (in gray; n = 71, mTTC = 7.46). Statistical significance was assessed by a linear-model regression analysis on the log-transformed mTTC values; ∗p = 0.05, ∗∗p = 0.01, and ∗∗∗p < 0.001.