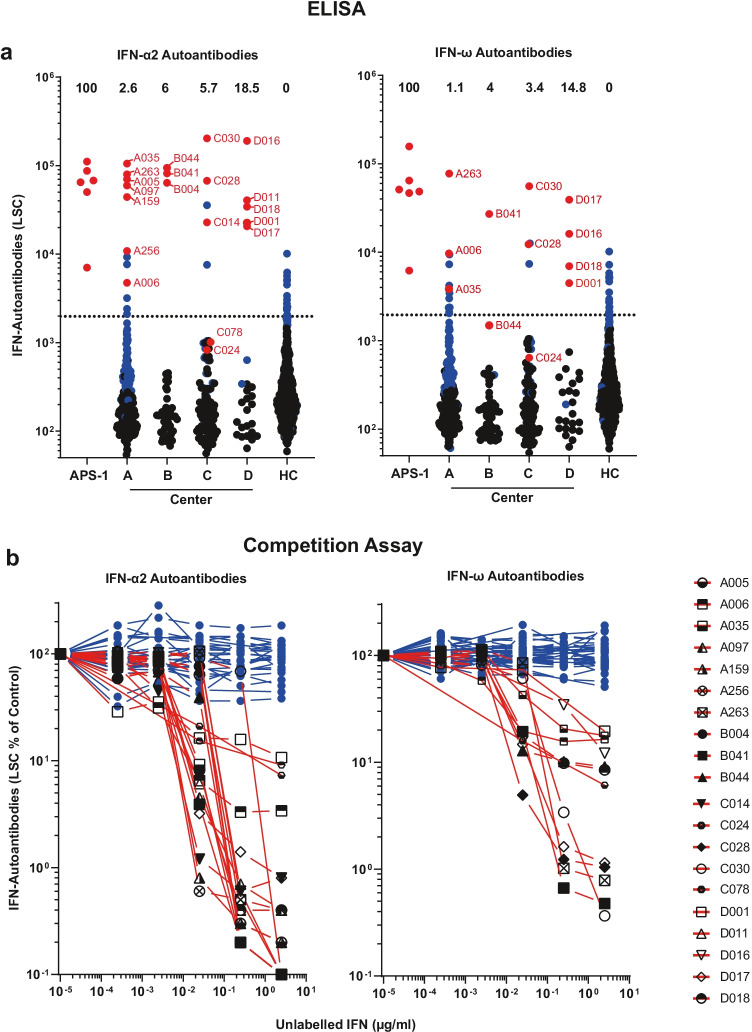
Fig. 1.
Prevalence of AABs against IFN-α2 and IFN-ω in patients with COVID-19. a ECLIA-based assay for detection of IgG AABs against IFN-α2 and IFN-ω in sera from hospitalized patients with COVID-19 from four different university hospital cohorts (Center A, n = 266; Center B, n = 50; Center C, n = 87; Center D, n = 27), in patients with APS-1 (n = 6), and healthy health care workers (HC) without documented SARS-CoV-2 infection (n = 667). Dotted lines indicate the 97.5th percentile of the ECLIA assay LSC in sera from the HC cohort. Dots indicate samples containing AABs scoring specific (red) or unspecific (blue) for IFN-α2 and IFN-ω binding in the competition assay (see b), respectively. Samples depicted as black dots were not tested in the competition assay. The prevalence of sera with specifically binding type I IFN-AABs in each cohort is given in percent. b Specificity of the ECLIA assay signal for IFN-α2- and IFN-ω-AABs was tested in an competition assay by preincubation of sera with increasing concentrations of unlabeled IFN-α2 and IFN-ω protein (0–2.5 µg/ml) before analysis. Samples showing a decrease in assay signal by at least 75% in the presence of the highest competitor concentration were defined as specific for type I IFN antibody reactivity and are indicated with red lines (IFN-α2 n = 20, IFN-ω n = 12). Samples showing no decrease in the presence of excess unlabeled type I IFN protein were regarded as unspecific for type I IFN antibody reactivity and are indicated with blue lines (IFN-α2 n = 62, IFN-ω n = 39)