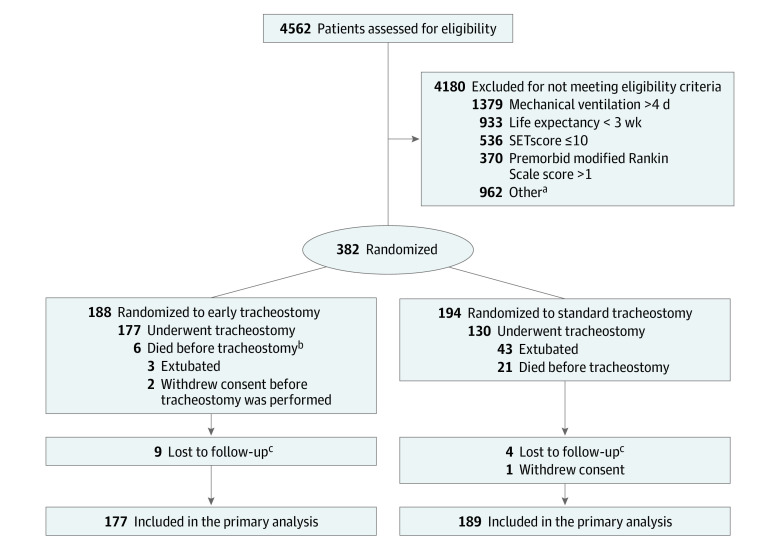
Figure 1. Randomization and Follow-up of Patients in the SETPOINT2 Trial.
aTwo hundred ninety-four were not considered appropriate study candidates, 257 did not consent or had no legal proxy, 156 were participating in another trial, 96 had critical illness such that early tracheostomy could have compromised well-being, 64 were expected to need a permanent surgical tracheostomy, 14 had language barriers, 13 did not pursue maximal intensive care therapy, 9 required ventilation unrelated to stroke, 4 were admitted when tracheostomy interventionalists were unavailable, and 55 patients had other reasons.
bDied before planned tracheostomy or deterioration of clinical condition prevented tracheostomy at the planned time and ultimately died.
cCould not be reached or found for the telephone follow-up.
SET indicates stroke-related early tracheostomy (range, 0-37; ≥10 estimates ≥2 weeks of ventilatory support).