The title polyoxometalate salt, (NH4)2[Zn(DMF)(H2O)5]2[V10O28]·4H2O, comprising a cationic Zn2+ complex, belongs to a group of relatively rare decavanadates with complex counter-ions, previously including only eight crystal structures with zinc(II) components. In the crystal structure, the ions are linked by strong O—H⋯O hydrogen bonds between the coordinated water ligands and the decavanadate, with the O⋯O distances ranging from 2.660 (2) to 2.893 (2) Å.
Keywords: crystal structure, decavanadate, zinc complexes, polyoxometalate, hydrogen bonds
Abstract
The crystalline product (NH4)2[Zn(C3H7NO)(H2O)5]2[V10O28]·4H2O was successfully isolated from an H2O/DMF solvent combination by evaporation at ambient temperature. The salt crystallizes in the P21/n space group. Imidazole, initially used in the synthesis but not present in the product, and DMF solvent appear to affect the synthesis and crystallization as structural-directing agents. In the title compound, the complex cation [Zn(H2O)5(DMF)]2+ acts as a counter-ion without being directly coordinated to the decavanadate anion. An extensive framework of hydrogen bonds integrates the whole architecture as evidenced by X-ray crystallography. The polyoxometalate [V10O28]6– lies on a center of symmetry while the complex cation [Zn(H2O)5(DMF)]2+ links three adjacent anions through a set of 2 + 2 + 3 hydrogen bonds.
1. Chemical context
Decavanadate anions, H
x
V10O28
(6–x)–, are the major species in equilibrated aqueous vanadate solutions (Rehder, 2015 ▸; Gorzsás et al., 2009 ▸) at vanadium(V) concentrations above 1 mM in the pH range of ≃2–6 (Schmidt et al., 2001 ▸; Pettersson et al., 1985 ▸), and are also stabilized in some organic solvents (Slebodnick & Pecoraro, 1998 ▸). There are altogether 54 compounds in the CSD (WebCSD, accessed January 2022; Groom et al., 2016 ▸) that contain a decavanadate anion and a transition-metal complex cation, either coordinated or as a free counter-ion. Both groups are evenly abundant (27 structures). In our search for conditions under which the decavanadate acts as a ligand we focused on Zn2+ complexes that have already shown the ability to act as a counter-ion: (NH4)2[Zn (H2O)6]2[V10O28]·4H2O (Udomvech et al., 2012 ▸), [Zn(H2O)6]
n
[{Na2(H2O)6(μ2-H2O)4Zn(H2O)2}V10O28]
n
·4nH2O (Yerra & Das, 2017 ▸), [Zn3(Htrz)6(H2O)6][V10O28]·10H2O·Htrz (Xu et al., 2012 ▸), (C4H14N2)2]·[Zn(H2O)6][V10O28]·6H2O (Jin et al., 2018 ▸), (NH4)2[Zn(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O (Klištincová et al., 2010 ▸); as well as being directly coordinated to decavanadate: {[Zn2(H2O)14[V10O28]}·H2
ppz (Wang et al., 2008 ▸), {[Zn(en)2]3[V10O28]}·5H2O (Pang et al., 2012 ▸), {[Zn(im)2(DMF)2]2[H2V10O28]·im·DMF (Xu et al., 2012 ▸), {[Zn3(trz)3(H2O)4(DMF)]2[V10O28]·4H2O}
n
(Xu et al., 2012 ▸), [(CH3)4N]2[Zn(H2O)5]2[V10O28]}·5H2O (Huang et al., 2021 ▸) and {[Zn(H2O)6][Zn2[V10O28](H2O)10]}·6H2O (Graia et al., 2008 ▸) (im = imidazole, Htrz = 1,2,4-triazole, DMF = N,N′-dimethylformamide, en = ethane-1,2-diamine, ppz = piperazine). Employing zinc(II) centers as part of linker moieties for the construction of polyoxometalate-based metal organic frameworks (POMOFs) comes with an advantage over traditionally used rare metals regarding costs, and sometimes even efficiency. Important applications of POMOFs in materials chemistry include, for instance, photovoltaics (Luo et al., 2012 ▸) and hydrogen evolution (Nohra et al., 2011 ▸). Despite extensive experimental work with an inexpensive multicomponent system H2O/DMF/imidazole/Zn2+/V5+, we were not able to isolate from the various preparations any crystalline product other than (NH4)2[Zn(H2O)5(DMF)]2[V10O28]·4H2O (1). Its crystal structure is presented here.
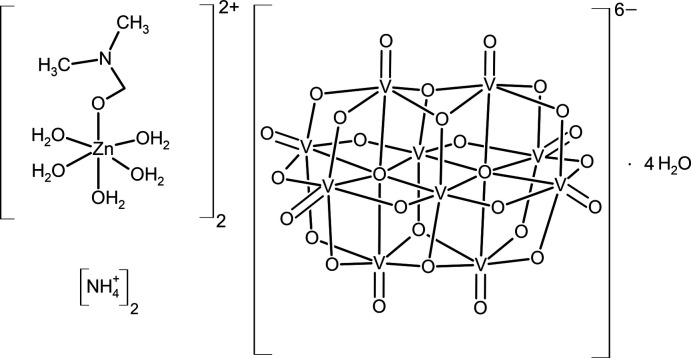
2. Structural commentary
Compound 1 crystallizes from a bicomponent solvent H2O/DMF at room temperature in the form of orange block-shaped crystals in monoclinic symmetry [P21/n; β = 108.628 (1)°]. Although imidazole is not present in the crystal structure, neither as a free molecule or cation nor as a ligand, its presence was necessary for crystallization to take place. In the absence of imidazole we observed the formation of oily solutions without crystalline product or the slow reduction of vanadium accompanied by a change in color of the solution from orange to greenish. The asymmetric unit of (NH4)2[Zn(H2O)5(DMF)]2[V10O28]·4H2O (Fig. 1 ▸) comprises one half of the [V10O28]6– polyoxometalate, one [Zn(H2O)5(DMF)]2+ complex cation, one NH4 + and two molecules of water of crystallization. The H atoms of the ammonium cation and water molecules were found in the difference map and refined freely except for three water molecules where restraints on the O—H distances were applied. The H atoms bound to the C atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms. The Zn2+ center in [Zn(H2O)5(DMF)]2+ is coordinated by five aqua ligands with Zn—O bond lengths in the range 2.0482 (16)–2.1273 (16) Å and one N,N′-dimethylformamide ligand coordinated through the oxygen atom with a Zn—O bond length of 2.0926 (14) Å, forming an irregular octahedron. The decavanadate anion [V10O28]6– is present in a fully deprotonated form, as further confirmed by elemental analysis and charge balance. It resides in a special position on the center of symmetry, as observed many times before (Rakovský & Krivosudský, 2014 ▸). The anion adopts C i symmetry (idealized D 2h ) and is composed of ten edge-sharing heavily distorted octahedra. The terminal vanadium–oxygen bond lengths (V=O groups) are in the range 1.5929 (14)–1.6210 (14) Å, with an average value of 1.6083 Å. The bond lengths of the bridging μ–O atoms are in the range 1.6890 (13)–2.0696 (14) Å, with an average value of 1.853 Å. The bond lengths of the bridging μ 3–O atoms with coordination numbers of three are in the range 1.8700 (14)–2.0208 (14) Å, with an average value of 1.9725 Å. Bond lengths of the hexacoordinated oxygen atom trapped inside the decavanadate (O16) are in the range 2.1033 (13)–2.3337 (13) Å, with an average value of 2.2222 Å. All metrical parameters fall in their typical ranges.
Figure 1.
The molecular structure of 1 showing 50% displacement ellipsoids illustrated with DIAMOND (Brandenburg & Putz, 2005 ▸). The half of the decavanadate anion that is not part of the asymmetric unit is displayed as faded.
3. Supramolecular features
The supramolecular structure of 1 is stabilized by a rich network of hydrogen bonds that involves all components of the compound. The strongest hydrogen bonds are formed by the complex cation (Fig. 2 ▸, Table 1 ▸), which serves as a linker for decavanadate anions in its vicinity. More specifically, [Zn(H2O)5(DMF)]2+ forms 2 + 2 + 3 hydrogen bonds through its aqua ligands (as donors) to three different [V10O28]6− anions (as acceptors). The structural parameters of the hydrogen bonds are summarized in Table 1 ▸. Based on the D⋯A distances ranging from 2.659 (2) to 2.892 (2) Å and the angles D—H⋯A falling into the range 164 (3)–177 (3)°, the hydrogen bonds may be considered relatively strong examples.
Figure 2.
Relative positions of the three adjacent decavanadate anions (orange polyhedra) linked by a single [Zn(H2O)5(DMF)]2+ cation.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2O⋯O15i | 0.75 (3) | 1.98 (3) | 2.722 (2) | 167 (3) |
| O3—H3P⋯O18 | 0.83 (3) | 2.07 (3) | 2.892 (2) | 173 (3) |
| O4—H4O⋯O17ii | 0.74 (3) | 1.97 (3) | 2.710 (2) | 177 (3) |
| O5—H5O⋯O19 | 0.85 (3) | 1.82 (3) | 2.659 (2) | 169 (3) |
| O5—H5P⋯O9iii | 0.76 (3) | 2.10 (3) | 2.842 (2) | 164 (3) |
| O6—H6O⋯O7iii | 0.82 (3) | 1.95 (3) | 2.771 (2) | 173 (3) |
| O6—H6P⋯O11ii | 0.78 (2) | 2.00 (2) | 2.769 (2) | 170 (3) |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 .
.
4. Database survey
In a search of the Cambridge Structural Database (WebCSD, accessed January 2022; Groom et al., 2016 ▸) for closely related decavanadates bearing mononuclear zinc(II) complex cations which are not coordinated to the decavanadate anion, six entries were found: (NH4)2[Zn(H2O)6]2[V10O28]·4H2O ICSD Entry: 422816 (Udomvech et al., 2012 ▸), [Zn(H2O)6] n [{Na2(H2O)6(μ-H2O)4Zn(H2O)2}V10O28] n ·4nH2O ICSD Entry: 427974 (Yerra & Das, 2017 ▸), (C4H14N2)2]·[Zn(H2O)6][V10O28]·6H2O YEYYEJ (Jin et al., 2018 ▸), (NH4)2[Zn(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O XABQIC (Klištincová et al., 2010 ▸), [Zn(3-Hdpye)(H2O)5]2[V10O28]·4H2O OXUYUD (Wang et al., 2016 ▸), and [Zn(H2O)6][Na3(H2O)14][HV10O28]·4H2O SUDGUW (Amanchi & Das, 2018 ▸). The overall compositions (cations, decavanadate anion, water) are in all cases similar to that of the title compound.
5. Synthesis and crystallization
NH4VO3 (0.464 g, 4 mmol) was dissolved in 20 ml of water and stirred upon heating. After being cooled down to ambient temperature, decavanadate was prepared in situ by adjusting the pH to 4 with 2 M HCl until the color of the solution changed from bright yellow to orange. Under continuous stirring, imidazole (0.136 g, 2 mmol) was poured into the mixture and the pH was adjusted to 4 by adding 2 M HCl again. Finally, first ZnSO4·7H2O (0.287 g, 1 mmol) and secondly 20 mL of DMF were added to the clear solution. The mixture was filtered, and the clear orange filtrate was left to crystallize at RT. The orange crystals were isolated a few days later. The vanadium content was determined using an ICP MS Thermo Scientific iCap-Q; the zinc content was determined using an AAS Perkin-Elmer Model 1100. An infrared spectrum was recorded on a Nicolet FTIR 6700 spectrometer in Nujol mull. Analytical data for C6H50N4O44V10Zn2: theoretical V 33.5%, Zn 8.6%; found V 32.4%, Zn 8.40%. Characteristic bands in the FTIR spectrum (in cm−1): V10O28 964, 951, 938, 805, 596; NH4 + 1416; DMF 1658, 1382, 1118.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined isotropically and those on carbon atoms were placed in geometrically idealized positions (C—H = 0.93 Å) and constrained to ride on their parent atoms with U iso(H) = 1.2 U eq(C). Hydrogen atoms of the water molecules and the ammonium cation were found in the difference-Fourier map. For the two lattice water molecules and one coordinated water, the O—H distances were restrained with DFIX while orientation and displacement parameters were refined freely. All other water hydrogen atoms and the ammonium cation hydrogen atoms were refined freely.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | (NH4)2[Zn(C3H7NO)(H2O)5]2[V10O28]·4H2O |
| M r | 1522.64 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 120 |
| a, b, c (Å) | 15.5436 (6), 8.6538 (4), 16.7362 (7) |
| β (°) | 108.628 (1) |
| V (Å3) | 2133.27 (16) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 3.31 |
| Crystal size (mm) | 0.49 × 0.23 × 0.10 |
| Data collection | |
| Diffractometer | Nonius KappaCCD with Buker APEXII detector |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.57, 0.73 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 29908, 4901, 4354 |
| R int | 0.033 |
| (sin θ/λ)max (Å−1) | 0.650 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.023, 0.056, 1.06 |
| No. of reflections | 4901 |
| No. of parameters | 372 |
| No. of restraints | 6 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.36, −0.62 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022003449/yz2017sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022003449/yz2017Isup2.hkl
CCDC reference: 2156016
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| (NH4)2[Zn(C3H7NO)(H2O)5]2[V10O28]·4H2O | F(000) = 1512 |
| Mr = 1522.64 | Dx = 2.370 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.5436 (6) Å | Cell parameters from 9949 reflections |
| b = 8.6538 (4) Å | θ = 2.6–27.5° |
| c = 16.7362 (7) Å | µ = 3.31 mm−1 |
| β = 108.628 (1)° | T = 120 K |
| V = 2133.27 (16) Å3 | Prism, orange |
| Z = 2 | 0.49 × 0.23 × 0.10 mm |
Data collection
| Nonius KappaCCD with Buker APEXII detector diffractometer | 4354 reflections with I > 2σ(I) |
| data from phi and ω scans | Rint = 0.033 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 27.5°, θmin = 2.2° |
| Tmin = 0.57, Tmax = 0.73 | h = −20→19 |
| 29908 measured reflections | k = −11→11 |
| 4901 independent reflections | l = −21→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.023 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.056 | w = 1/[σ2(Fo2) + (0.0284P)2 + 1.1852P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.002 |
| 4901 reflections | Δρmax = 0.36 e Å−3 |
| 372 parameters | Δρmin = −0.62 e Å−3 |
| 6 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Zn1 | 0.31800 (2) | 0.46454 (3) | 0.54363 (2) | 0.00759 (6) | |
| O1 | 0.36049 (10) | 0.62987 (16) | 0.47301 (9) | 0.0114 (3) | |
| O2 | 0.31034 (13) | 0.30400 (19) | 0.45168 (11) | 0.0159 (3) | |
| H2O | 0.276 (2) | 0.240 (3) | 0.4386 (19) | 0.027 (9)* | |
| H2P | 0.350 (2) | 0.281 (4) | 0.439 (2) | 0.043 (11)* | |
| O3 | 0.44977 (11) | 0.3899 (2) | 0.60794 (10) | 0.0125 (3) | |
| H3O | 0.4493 (17) | 0.310 (3) | 0.6204 (16) | 0.012 (7)* | |
| H3P | 0.478 (2) | 0.433 (3) | 0.653 (2) | 0.027 (8)* | |
| O4 | 0.27068 (12) | 0.31855 (17) | 0.62168 (10) | 0.0107 (3) | |
| H4O | 0.2351 (18) | 0.260 (3) | 0.6034 (16) | 0.012 (7)* | |
| H4P | 0.311 (2) | 0.274 (3) | 0.6540 (19) | 0.026 (8)* | |
| O5 | 0.32040 (12) | 0.64584 (19) | 0.62565 (10) | 0.0147 (3) | |
| H5O | 0.338 (2) | 0.648 (4) | 0.679 (2) | 0.037 (9)* | |
| H5P | 0.309 (2) | 0.728 (4) | 0.609 (2) | 0.041 (10)* | |
| O6 | 0.17863 (11) | 0.51996 (18) | 0.48529 (10) | 0.0107 (3) | |
| H6O | 0.1613 (18) | 0.600 (3) | 0.4584 (16) | 0.025 (8)* | |
| H6P | 0.1389 (16) | 0.463 (3) | 0.4648 (17) | 0.018 (7)* | |
| C1 | 0.31089 (15) | 0.6545 (2) | 0.39870 (13) | 0.0127 (4) | |
| H1 | 0.267624 | 0.577353 | 0.372375 | 0.015* | |
| N1 | 0.31404 (12) | 0.7783 (2) | 0.35441 (11) | 0.0126 (4) | |
| C2 | 0.37449 (17) | 0.9071 (3) | 0.38953 (16) | 0.0207 (5) | |
| H2A | 0.339767 | 0.991473 | 0.403582 | 0.031* | |
| H2B | 0.402328 | 0.943400 | 0.348019 | 0.031* | |
| H2C | 0.422078 | 0.873260 | 0.440631 | 0.031* | |
| C3 | 0.24580 (16) | 0.8028 (3) | 0.27197 (15) | 0.0230 (5) | |
| H3A | 0.208105 | 0.710072 | 0.255621 | 0.035* | |
| H3B | 0.276127 | 0.823842 | 0.229986 | 0.035* | |
| H3C | 0.207451 | 0.891060 | 0.275052 | 0.035* | |
| V1 | 0.66396 (2) | 0.54542 (4) | 0.97966 (2) | 0.00519 (8) | |
| V2 | 0.48442 (2) | 0.68753 (4) | 0.99591 (2) | 0.00468 (8) | |
| V3 | 0.54965 (2) | 0.33633 (4) | 0.83349 (2) | 0.00615 (8) | |
| V4 | 0.37025 (2) | 0.46974 (4) | 0.84932 (2) | 0.00550 (8) | |
| V5 | 0.51936 (2) | 0.68687 (4) | 0.82367 (2) | 0.00627 (8) | |
| O7 | 0.63516 (9) | 0.70057 (15) | 0.90117 (8) | 0.0068 (3) | |
| O8 | 0.48640 (9) | 0.80713 (15) | 0.91677 (8) | 0.0066 (3) | |
| O9 | 0.76998 (9) | 0.57672 (15) | 1.03142 (9) | 0.0079 (3) | |
| O10 | 0.60927 (9) | 0.67399 (15) | 1.05199 (8) | 0.0057 (3) | |
| O11 | 0.45500 (9) | 0.79640 (15) | 1.06789 (8) | 0.0066 (3) | |
| O12 | 0.57404 (10) | 0.20530 (16) | 0.77742 (9) | 0.0102 (3) | |
| O13 | 0.42410 (9) | 0.32537 (15) | 0.79962 (8) | 0.0067 (3) | |
| O14 | 0.26409 (10) | 0.43520 (16) | 0.80457 (9) | 0.0096 (3) | |
| O15 | 0.66637 (9) | 0.39324 (15) | 0.90763 (8) | 0.0068 (3) | |
| O16 | 0.51531 (9) | 0.50621 (14) | 0.92593 (8) | 0.0055 (3) | |
| O17 | 0.36261 (9) | 0.60759 (15) | 0.94154 (8) | 0.0061 (3) | |
| O18 | 0.54495 (10) | 0.51674 (15) | 0.77235 (9) | 0.0072 (3) | |
| O19 | 0.39504 (9) | 0.63135 (15) | 0.79227 (8) | 0.0070 (3) | |
| O20 | 0.51633 (10) | 0.82531 (16) | 0.75840 (9) | 0.0107 (3) | |
| N2 | 0.13802 (14) | 0.5436 (2) | 0.65462 (12) | 0.0091 (4) | |
| H2R | 0.0918 (19) | 0.476 (3) | 0.6319 (17) | 0.017 (7)* | |
| H2S | 0.176 (2) | 0.502 (3) | 0.691 (2) | 0.024 (8)* | |
| H2T | 0.1165 (18) | 0.620 (3) | 0.6764 (16) | 0.019 (7)* | |
| H2Q | 0.165 (2) | 0.572 (3) | 0.618 (2) | 0.031 (8)* | |
| O21 | 0.41424 (12) | 0.08923 (19) | 0.64987 (12) | 0.0191 (4) | |
| H21A | 0.446 (2) | 0.032 (3) | 0.6837 (18) | 0.036 (9)* | |
| H21B | 0.3783 (19) | 0.042 (3) | 0.6175 (18) | 0.037 (10)* | |
| O22 | 0.46542 (13) | 0.2601 (2) | 0.41289 (12) | 0.0260 (4) | |
| H22A | 0.472 (2) | 0.250 (4) | 0.3693 (16) | 0.040 (10)* | |
| H22B | 0.5092 (18) | 0.296 (4) | 0.4460 (18) | 0.039 (10)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.00799 (12) | 0.00689 (11) | 0.00765 (11) | −0.00058 (9) | 0.00214 (9) | −0.00008 (9) |
| O1 | 0.0120 (8) | 0.0121 (7) | 0.0107 (7) | −0.0031 (6) | 0.0046 (6) | 0.0005 (6) |
| O2 | 0.0122 (9) | 0.0163 (8) | 0.0222 (9) | −0.0065 (7) | 0.0099 (7) | −0.0108 (7) |
| O3 | 0.0122 (8) | 0.0103 (8) | 0.0122 (8) | 0.0005 (7) | 0.0001 (6) | 0.0005 (7) |
| O4 | 0.0087 (8) | 0.0085 (7) | 0.0129 (8) | −0.0020 (7) | 0.0006 (7) | 0.0017 (6) |
| O5 | 0.0246 (9) | 0.0090 (8) | 0.0068 (8) | 0.0014 (7) | −0.0002 (7) | −0.0004 (6) |
| O6 | 0.0079 (8) | 0.0077 (7) | 0.0133 (8) | −0.0006 (6) | −0.0009 (6) | 0.0015 (6) |
| C1 | 0.0122 (11) | 0.0143 (10) | 0.0135 (10) | 0.0001 (9) | 0.0069 (9) | −0.0010 (8) |
| N1 | 0.0106 (9) | 0.0150 (9) | 0.0142 (9) | 0.0021 (7) | 0.0069 (8) | 0.0037 (7) |
| C2 | 0.0288 (14) | 0.0143 (11) | 0.0244 (12) | −0.0034 (10) | 0.0162 (11) | 0.0011 (9) |
| C3 | 0.0163 (12) | 0.0374 (14) | 0.0168 (12) | 0.0070 (11) | 0.0073 (10) | 0.0110 (11) |
| V1 | 0.00446 (17) | 0.00613 (15) | 0.00523 (15) | 0.00007 (12) | 0.00188 (13) | −0.00022 (12) |
| V2 | 0.00508 (17) | 0.00396 (15) | 0.00497 (15) | 0.00072 (12) | 0.00156 (13) | 0.00013 (12) |
| V3 | 0.00678 (17) | 0.00668 (16) | 0.00561 (16) | 0.00051 (13) | 0.00282 (13) | −0.00102 (12) |
| V4 | 0.00511 (17) | 0.00675 (15) | 0.00438 (15) | 0.00021 (12) | 0.00115 (13) | −0.00009 (12) |
| V5 | 0.00768 (18) | 0.00618 (16) | 0.00514 (16) | 0.00000 (13) | 0.00230 (13) | 0.00097 (12) |
| O7 | 0.0071 (7) | 0.0072 (6) | 0.0068 (6) | −0.0008 (5) | 0.0032 (6) | −0.0003 (5) |
| O8 | 0.0068 (7) | 0.0058 (6) | 0.0074 (7) | 0.0003 (5) | 0.0024 (6) | 0.0003 (5) |
| O9 | 0.0070 (7) | 0.0088 (6) | 0.0081 (7) | 0.0001 (6) | 0.0027 (6) | 0.0000 (5) |
| O10 | 0.0057 (7) | 0.0055 (6) | 0.0056 (6) | −0.0004 (5) | 0.0011 (5) | −0.0006 (5) |
| O11 | 0.0068 (7) | 0.0061 (6) | 0.0072 (7) | 0.0007 (5) | 0.0027 (6) | 0.0000 (5) |
| O12 | 0.0104 (8) | 0.0113 (7) | 0.0097 (7) | 0.0000 (6) | 0.0045 (6) | −0.0033 (6) |
| O13 | 0.0066 (7) | 0.0076 (6) | 0.0061 (6) | 0.0000 (5) | 0.0023 (6) | −0.0003 (5) |
| O14 | 0.0078 (7) | 0.0121 (7) | 0.0081 (7) | −0.0005 (6) | 0.0014 (6) | 0.0002 (6) |
| O15 | 0.0060 (7) | 0.0077 (6) | 0.0075 (7) | 0.0001 (5) | 0.0033 (6) | 0.0000 (5) |
| O16 | 0.0045 (7) | 0.0057 (6) | 0.0063 (6) | 0.0001 (5) | 0.0019 (6) | 0.0004 (5) |
| O17 | 0.0045 (7) | 0.0062 (6) | 0.0075 (6) | 0.0009 (5) | 0.0019 (5) | 0.0002 (5) |
| O18 | 0.0071 (7) | 0.0084 (6) | 0.0064 (6) | −0.0006 (5) | 0.0029 (6) | −0.0002 (5) |
| O19 | 0.0074 (7) | 0.0079 (6) | 0.0055 (6) | 0.0012 (5) | 0.0017 (6) | 0.0014 (5) |
| O20 | 0.0117 (8) | 0.0109 (7) | 0.0095 (7) | −0.0005 (6) | 0.0033 (6) | 0.0018 (6) |
| N2 | 0.0090 (9) | 0.0078 (8) | 0.0095 (9) | 0.0005 (8) | 0.0014 (8) | 0.0011 (7) |
| O21 | 0.0162 (9) | 0.0113 (8) | 0.0217 (9) | −0.0007 (7) | −0.0056 (8) | −0.0002 (7) |
| O22 | 0.0184 (10) | 0.0405 (11) | 0.0223 (10) | −0.0112 (8) | 0.0110 (9) | −0.0143 (9) |
Geometric parameters (Å, º)
| Zn1—O2 | 2.0482 (16) | V2—O11 | 1.7029 (14) |
| Zn1—O5 | 2.0772 (16) | V2—O10 | 1.8700 (14) |
| Zn1—O3 | 2.0886 (16) | V2—O17 | 1.9470 (14) |
| Zn1—O1 | 2.0926 (14) | V2—O16 | 2.1033 (13) |
| Zn1—O4 | 2.1113 (16) | V2—O16i | 2.1256 (13) |
| Zn1—O6 | 2.1273 (16) | V2—V3i | 3.0726 (5) |
| O1—C1 | 1.255 (3) | V2—V5 | 3.0993 (5) |
| O2—H2O | 0.75 (3) | V3—O12 | 1.5929 (14) |
| O2—H2P | 0.74 (3) | V3—O13 | 1.8525 (14) |
| O3—H3O | 0.73 (3) | V3—O18 | 1.8552 (14) |
| O3—H3P | 0.83 (3) | V3—O15 | 1.9067 (14) |
| O4—H4O | 0.74 (3) | V3—O11i | 2.0310 (14) |
| O4—H4P | 0.79 (3) | V3—O16 | 2.3168 (14) |
| O5—H5O | 0.84 (3) | V3—V5 | 3.0662 (5) |
| O5—H5P | 0.76 (3) | V3—V4 | 3.1066 (5) |
| O6—H6O | 0.82 (2) | V4—O14 | 1.6074 (15) |
| O6—H6P | 0.78 (2) | V4—O19 | 1.8031 (14) |
| C1—N1 | 1.313 (3) | V4—O13 | 1.8434 (14) |
| C1—H1 | 0.9500 | V4—O17 | 1.9841 (14) |
| N1—C2 | 1.455 (3) | V4—O10i | 2.0101 (14) |
| N1—C3 | 1.463 (3) | V4—O16 | 2.2317 (14) |
| C2—H2A | 0.9800 | V4—V5 | 3.1181 (5) |
| C2—H2B | 0.9800 | V5—O20 | 1.6118 (14) |
| C2—H2C | 0.9800 | V5—O18 | 1.8115 (14) |
| C3—H3A | 0.9800 | V5—O7 | 1.8559 (14) |
| C3—H3B | 0.9800 | V5—O19 | 1.8954 (14) |
| C3—H3C | 0.9800 | V5—O8 | 2.0696 (14) |
| V1—O9 | 1.6210 (14) | V5—O16 | 2.3337 (13) |
| V1—O15 | 1.7939 (14) | N2—H2R | 0.91 (3) |
| V1—O7 | 1.8312 (14) | N2—H2S | 0.79 (3) |
| V1—O17i | 2.0027 (14) | N2—H2T | 0.87 (3) |
| V1—O10 | 2.0208 (14) | N2—H2Q | 0.88 (3) |
| V1—O16 | 2.2215 (14) | O21—H21A | 0.79 (2) |
| V1—V4i | 3.0765 (5) | O21—H21B | 0.76 (2) |
| V1—V5 | 3.1011 (5) | O22—H22A | 0.78 (2) |
| V1—V3 | 3.1047 (5) | O22—H22B | 0.79 (2) |
| V2—O8 | 1.6890 (13) | ||
| O2—Zn1—O5 | 173.36 (7) | O11i—V3—V2i | 31.29 (4) |
| O2—Zn1—O3 | 89.40 (7) | O16—V3—V2i | 43.72 (3) |
| O5—Zn1—O3 | 94.90 (7) | V5—V3—V2i | 92.705 (12) |
| O2—Zn1—O1 | 89.57 (7) | O12—V3—V1 | 134.03 (5) |
| O5—Zn1—O1 | 85.10 (6) | O13—V3—V1 | 123.55 (4) |
| O3—Zn1—O1 | 93.90 (6) | O18—V3—V1 | 81.71 (4) |
| O2—Zn1—O4 | 96.37 (7) | O15—V3—V1 | 31.85 (4) |
| O5—Zn1—O4 | 88.82 (6) | O11i—V3—V1 | 81.33 (4) |
| O3—Zn1—O4 | 88.52 (7) | O16—V3—V1 | 45.57 (3) |
| O1—Zn1—O4 | 173.62 (6) | V5—V3—V1 | 60.334 (11) |
| O2—Zn1—O6 | 90.11 (7) | V2i—V3—V1 | 62.101 (11) |
| O5—Zn1—O6 | 86.20 (6) | O12—V3—V4 | 134.74 (5) |
| O3—Zn1—O6 | 173.47 (6) | O13—V3—V4 | 32.71 (4) |
| O1—Zn1—O6 | 92.61 (6) | O18—V3—V4 | 81.72 (4) |
| O4—Zn1—O6 | 85.06 (6) | O15—V3—V4 | 122.81 (4) |
| C1—O1—Zn1 | 118.11 (14) | O11i—V3—V4 | 82.82 (4) |
| Zn1—O2—H2O | 126 (2) | O16—V3—V4 | 45.79 (3) |
| Zn1—O2—H2P | 123 (3) | V5—V3—V4 | 60.676 (11) |
| H2O—O2—H2P | 107 (3) | V2i—V3—V4 | 61.307 (10) |
| Zn1—O3—H3O | 110 (2) | V1—V3—V4 | 91.121 (12) |
| Zn1—O3—H3P | 118 (2) | O14—V4—O19 | 104.99 (7) |
| H3O—O3—H3P | 103 (3) | O14—V4—O13 | 102.14 (7) |
| Zn1—O4—H4O | 121 (2) | O19—V4—O13 | 94.71 (6) |
| Zn1—O4—H4P | 111 (2) | O14—V4—O17 | 99.59 (6) |
| H4O—O4—H4P | 107 (3) | O19—V4—O17 | 91.24 (6) |
| Zn1—O5—H5O | 130 (2) | O13—V4—O17 | 155.11 (6) |
| Zn1—O5—H5P | 121 (2) | O14—V4—O10i | 97.90 (7) |
| H5O—O5—H5P | 108 (3) | O19—V4—O10i | 155.58 (6) |
| Zn1—O6—H6O | 123.2 (19) | O13—V4—O10i | 88.61 (6) |
| Zn1—O6—H6P | 127.5 (19) | O17—V4—O10i | 76.46 (5) |
| H6O—O6—H6P | 102 (3) | O14—V4—O16 | 172.94 (6) |
| O1—C1—N1 | 125.2 (2) | O19—V4—O16 | 81.20 (6) |
| O1—C1—H1 | 117.4 | O13—V4—O16 | 80.45 (6) |
| N1—C1—H1 | 117.4 | O17—V4—O16 | 76.62 (5) |
| C1—N1—C2 | 122.21 (19) | O10i—V4—O16 | 75.50 (5) |
| C1—N1—C3 | 120.29 (19) | O14—V4—V1i | 88.21 (5) |
| C2—N1—C3 | 116.75 (19) | O19—V4—V1i | 130.95 (4) |
| N1—C2—H2A | 109.5 | O13—V4—V1i | 128.99 (4) |
| N1—C2—H2B | 109.5 | O17—V4—V1i | 39.72 (4) |
| H2A—C2—H2B | 109.5 | O10i—V4—V1i | 40.38 (4) |
| N1—C2—H2C | 109.5 | O16—V4—V1i | 85.11 (4) |
| H2A—C2—H2C | 109.5 | O14—V4—V3 | 134.99 (5) |
| H2B—C2—H2C | 109.5 | O19—V4—V3 | 83.90 (4) |
| N1—C3—H3A | 109.5 | O13—V4—V3 | 32.89 (4) |
| N1—C3—H3B | 109.5 | O17—V4—V3 | 124.63 (4) |
| H3A—C3—H3B | 109.5 | O10i—V4—V3 | 85.94 (4) |
| N1—C3—H3C | 109.5 | O16—V4—V3 | 48.08 (3) |
| H3A—C3—H3C | 109.5 | V1i—V4—V3 | 119.349 (14) |
| H3B—C3—H3C | 109.5 | O14—V4—V5 | 138.20 (5) |
| O9—V1—O15 | 104.24 (7) | O19—V4—V5 | 33.46 (4) |
| O9—V1—O7 | 103.57 (7) | O13—V4—V5 | 83.20 (4) |
| O15—V1—O7 | 96.26 (6) | O17—V4—V5 | 88.66 (4) |
| O9—V1—O17i | 98.45 (6) | O10i—V4—V5 | 123.81 (4) |
| O15—V1—O17i | 90.58 (6) | O16—V4—V5 | 48.31 (3) |
| O7—V1—O17i | 154.50 (6) | V1i—V4—V5 | 120.716 (13) |
| O9—V1—O10 | 97.95 (6) | V3—V4—V5 | 59.021 (11) |
| O15—V1—O10 | 155.49 (6) | O20—V5—O18 | 104.25 (7) |
| O7—V1—O10 | 88.44 (6) | O20—V5—O7 | 103.77 (7) |
| O17i—V1—O10 | 75.81 (5) | O18—V5—O7 | 94.15 (6) |
| O9—V1—O16 | 171.99 (6) | O20—V5—O19 | 101.19 (7) |
| O15—V1—O16 | 81.84 (6) | O18—V5—O19 | 91.23 (6) |
| O7—V1—O16 | 80.63 (6) | O7—V5—O19 | 152.28 (6) |
| O17i—V1—O16 | 76.05 (5) | O20—V5—O8 | 100.13 (6) |
| O10—V1—O16 | 75.19 (5) | O18—V5—O8 | 155.54 (6) |
| O9—V1—V4i | 87.61 (5) | O7—V5—O8 | 81.94 (6) |
| O15—V1—V4i | 129.85 (4) | O19—V5—O8 | 81.98 (6) |
| O7—V1—V4i | 128.56 (4) | O20—V5—O16 | 173.31 (6) |
| O17i—V1—V4i | 39.28 (4) | O18—V5—O16 | 82.22 (5) |
| O10—V1—V4i | 40.12 (4) | O7—V5—O16 | 77.15 (5) |
| O16—V1—V4i | 84.47 (4) | O19—V5—O16 | 76.68 (5) |
| O9—V1—V5 | 136.35 (5) | O8—V5—O16 | 73.35 (5) |
| O15—V1—V5 | 83.63 (5) | O20—V5—V3 | 137.94 (5) |
| O7—V1—V5 | 32.99 (4) | O18—V5—V3 | 33.71 (4) |
| O17i—V1—V5 | 124.67 (4) | O7—V5—V3 | 85.86 (4) |
| O10—V1—V5 | 87.51 (4) | O19—V5—V3 | 83.66 (4) |
| O16—V1—V5 | 48.63 (3) | O8—V5—V3 | 121.86 (4) |
| V4i—V1—V5 | 120.389 (14) | O16—V5—V3 | 48.51 (3) |
| O9—V1—V3 | 138.31 (5) | O20—V5—V2 | 130.78 (5) |
| O15—V1—V3 | 34.12 (4) | O18—V5—V2 | 124.94 (5) |
| O7—V1—V3 | 85.11 (4) | O7—V5—V2 | 76.63 (4) |
| O17i—V1—V3 | 86.96 (4) | O19—V5—V2 | 78.01 (4) |
| O10—V1—V3 | 123.27 (4) | O8—V5—V2 | 30.65 (4) |
| O16—V1—V3 | 48.13 (4) | O16—V5—V2 | 42.72 (3) |
| V4i—V1—V3 | 118.865 (14) | V3—V5—V2 | 91.233 (12) |
| V5—V1—V3 | 59.218 (11) | O20—V5—V1 | 136.08 (5) |
| O8—V2—O11 | 106.98 (7) | O18—V5—V1 | 82.44 (5) |
| O8—V2—O10 | 98.98 (6) | O7—V5—V1 | 32.50 (4) |
| O11—V2—O10 | 98.61 (6) | O19—V5—V1 | 122.27 (4) |
| O8—V2—O17 | 96.31 (6) | O8—V5—V1 | 81.48 (4) |
| O11—V2—O17 | 94.96 (6) | O16—V5—V1 | 45.59 (3) |
| O10—V2—O17 | 155.59 (6) | V3—V5—V1 | 60.448 (11) |
| O8—V2—O16 | 87.47 (6) | V2—V5—V1 | 60.851 (11) |
| O11—V2—O16 | 165.32 (6) | O20—V5—V4 | 132.78 (5) |
| O10—V2—O16 | 81.25 (6) | O18—V5—V4 | 82.01 (5) |
| O17—V2—O16 | 80.53 (5) | O7—V5—V4 | 122.67 (4) |
| O8—V2—O16i | 165.68 (6) | O19—V5—V4 | 31.63 (4) |
| O11—V2—O16i | 87.09 (6) | O8—V5—V4 | 79.96 (4) |
| O10—V2—O16i | 81.02 (6) | O16—V5—V4 | 45.57 (3) |
| O17—V2—O16i | 79.50 (5) | V3—V5—V4 | 60.303 (11) |
| O16—V2—O16i | 78.36 (6) | V2—V5—V4 | 60.938 (10) |
| O8—V2—V3i | 145.25 (5) | V1—V5—V4 | 90.971 (12) |
| O11—V2—V3i | 38.27 (5) | V1—O7—V5 | 114.51 (7) |
| O10—V2—V3i | 89.35 (4) | V2—O8—V5 | 110.69 (7) |
| O17—V2—V3i | 88.85 (4) | V2—O10—V4i | 108.53 (6) |
| O16—V2—V3i | 127.24 (4) | V2—O10—V1 | 107.55 (6) |
| O16i—V2—V3i | 48.87 (4) | V4i—O10—V1 | 99.50 (6) |
| O8—V2—V5 | 38.66 (5) | V2—O11—V3i | 110.45 (7) |
| O11—V2—V5 | 145.64 (5) | V4—O13—V3 | 114.40 (7) |
| O10—V2—V5 | 90.27 (4) | V1—O15—V3 | 114.03 (7) |
| O17—V2—V5 | 89.87 (4) | V2—O16—V2i | 101.64 (6) |
| O16—V2—V5 | 48.82 (4) | V2—O16—V1 | 93.07 (5) |
| O16i—V2—V5 | 127.18 (4) | V2i—O16—V1 | 94.24 (5) |
| V3i—V2—V5 | 176.038 (14) | V2—O16—V4 | 93.27 (5) |
| O12—V3—O13 | 102.07 (7) | V2i—O16—V4 | 92.58 (5) |
| O12—V3—O18 | 104.42 (7) | V1—O16—V4 | 169.57 (7) |
| O13—V3—O18 | 91.28 (6) | V2—O16—V3 | 170.95 (7) |
| O12—V3—O15 | 102.19 (7) | V2i—O16—V3 | 87.41 (5) |
| O13—V3—O15 | 154.51 (6) | V1—O16—V3 | 86.30 (5) |
| O18—V3—O15 | 90.18 (6) | V4—O16—V3 | 86.13 (5) |
| O12—V3—O11i | 98.81 (6) | V2—O16—V5 | 88.46 (5) |
| O13—V3—O11i | 84.97 (6) | V2i—O16—V5 | 169.89 (7) |
| O18—V3—O11i | 156.74 (6) | V1—O16—V5 | 85.77 (5) |
| O15—V3—O11i | 83.72 (6) | V4—O16—V5 | 86.12 (5) |
| O12—V3—O16 | 173.76 (6) | V3—O16—V5 | 82.50 (4) |
| O13—V3—O16 | 77.99 (5) | V2—O17—V4 | 106.64 (6) |
| O18—V3—O16 | 81.80 (5) | V2—O17—V1i | 107.55 (6) |
| O15—V3—O16 | 77.04 (5) | V4—O17—V1i | 101.01 (6) |
| O11i—V3—O16 | 74.96 (5) | V5—O18—V3 | 113.48 (7) |
| O12—V3—V5 | 137.23 (5) | V4—O19—V5 | 114.91 (7) |
| O13—V3—V5 | 84.58 (4) | H2R—N2—H2S | 110 (3) |
| O18—V3—V5 | 32.81 (4) | H2R—N2—H2T | 108 (2) |
| O15—V3—V5 | 82.95 (4) | H2S—N2—H2T | 109 (3) |
| O11i—V3—V5 | 123.94 (4) | H2R—N2—H2Q | 112 (3) |
| O16—V3—V5 | 48.99 (3) | H2S—N2—H2Q | 104 (3) |
| O12—V3—V2i | 130.07 (5) | H2T—N2—H2Q | 114 (2) |
| O13—V3—V2i | 78.67 (4) | H21A—O21—H21B | 109 (3) |
| O18—V3—V2i | 125.52 (4) | H22A—O22—H22B | 111 (3) |
| O15—V3—V2i | 79.82 (4) | ||
| Zn1—O1—C1—N1 | 160.84 (16) | V1i—V4—O13—V3 | −84.80 (8) |
| O1—C1—N1—C2 | −3.0 (3) | V5—V4—O13—V3 | 39.62 (6) |
| O1—C1—N1—C3 | −172.8 (2) | O12—V3—O13—V4 | −177.51 (8) |
| O9—V1—O7—V5 | 174.37 (7) | O18—V3—O13—V4 | −72.47 (8) |
| O15—V1—O7—V5 | 68.06 (8) | O15—V3—O13—V4 | 20.64 (18) |
| O17i—V1—O7—V5 | −36.62 (17) | O11i—V3—O13—V4 | 84.54 (7) |
| O10—V1—O7—V5 | −87.82 (7) | O16—V3—O13—V4 | 8.87 (7) |
| O16—V1—O7—V5 | −12.58 (7) | V5—V3—O13—V4 | −40.31 (6) |
| V4i—V1—O7—V5 | −87.71 (8) | V2i—V3—O13—V4 | 53.56 (6) |
| V3—V1—O7—V5 | 35.75 (6) | V1—V3—O13—V4 | 8.43 (9) |
| O20—V5—O7—V1 | −174.70 (8) | O9—V1—O15—V3 | −177.41 (7) |
| O18—V5—O7—V1 | −68.93 (8) | O7—V1—O15—V3 | −71.66 (8) |
| O19—V5—O7—V1 | 31.69 (17) | O17i—V1—O15—V3 | 83.73 (7) |
| O8—V5—O7—V1 | 86.78 (7) | O10—V1—O15—V3 | 28.39 (18) |
| O16—V5—O7—V1 | 12.11 (7) | O16—V1—O15—V3 | 7.90 (7) |
| V3—V5—O7—V1 | −36.23 (6) | V4i—V1—O15—V3 | 83.63 (8) |
| V2—V5—O7—V1 | 56.03 (6) | V5—V1—O15—V3 | −41.12 (6) |
| V4—V5—O7—V1 | 14.23 (9) | O20—V5—O18—V3 | −178.51 (8) |
| O11—V2—O8—V5 | −179.02 (6) | O7—V5—O18—V3 | 76.15 (8) |
| O10—V2—O8—V5 | 79.05 (7) | O19—V5—O18—V3 | −76.64 (8) |
| O17—V2—O8—V5 | −81.86 (7) | O8—V5—O18—V3 | −3.5 (2) |
| O16—V2—O8—V5 | −1.67 (7) | O16—V5—O18—V3 | −0.26 (7) |
| O16i—V2—O8—V5 | −9.8 (3) | V2—V5—O18—V3 | −0.44 (10) |
| V3i—V2—O8—V5 | −178.97 (3) | V1—V5—O18—V3 | 45.77 (6) |
| O8—V2—O10—V4i | −178.45 (6) | V4—V5—O18—V3 | −46.29 (6) |
| O11—V2—O10—V4i | 72.71 (7) | O12—V3—O18—V5 | −179.26 (8) |
| O17—V2—O10—V4i | −50.33 (16) | O13—V3—O18—V5 | 77.94 (8) |
| O16—V2—O10—V4i | −92.44 (6) | O15—V3—O18—V5 | −76.62 (8) |
| O16i—V2—O10—V4i | −12.94 (6) | O11i—V3—O18—V5 | −2.3 (2) |
| V3i—V2—O10—V4i | 35.44 (5) | O16—V3—O18—V5 | 0.27 (7) |
| V5—V2—O10—V4i | −140.62 (5) | V2i—V3—O18—V5 | 0.97 (10) |
| O8—V2—O10—V1 | −71.68 (7) | V1—V3—O18—V5 | −45.80 (6) |
| O11—V2—O10—V1 | 179.48 (6) | V4—V3—O18—V5 | 46.56 (6) |
| O17—V2—O10—V1 | 56.44 (16) | O14—V4—O19—V5 | −174.03 (7) |
| O16—V2—O10—V1 | 14.33 (6) | O13—V4—O19—V5 | −70.12 (8) |
| O16i—V2—O10—V1 | 93.83 (6) | O17—V4—O19—V5 | 85.69 (8) |
| V3i—V2—O10—V1 | 142.21 (5) | O10i—V4—O19—V5 | 26.93 (18) |
| V5—V2—O10—V1 | −33.84 (5) | O16—V4—O19—V5 | 9.44 (7) |
| O8—V2—O11—V3i | 179.95 (7) | V1i—V4—O19—V5 | 85.04 (8) |
| O10—V2—O11—V3i | −77.85 (7) | V3—V4—O19—V5 | −39.01 (6) |
| O17—V2—O11—V3i | 81.80 (7) | O20—V5—O19—V4 | 177.33 (8) |
| O16—V2—O11—V3i | 10.5 (3) | O18—V5—O19—V4 | 72.55 (8) |
| O16i—V2—O11—V3i | 2.61 (7) | O7—V5—O19—V4 | −28.78 (17) |
| V5—V2—O11—V3i | 178.87 (3) | O8—V5—O19—V4 | −83.86 (8) |
| O14—V4—O13—V3 | 177.55 (8) | O16—V5—O19—V4 | −9.16 (7) |
| O19—V4—O13—V3 | 71.10 (8) | V3—V5—O19—V4 | 39.64 (6) |
| O17—V4—O13—V3 | −32.15 (18) | V2—V5—O19—V4 | −52.98 (6) |
| O10i—V4—O13—V3 | −84.67 (8) | V1—V5—O19—V4 | −9.28 (9) |
| O16—V4—O13—V3 | −9.14 (7) |
Symmetry code: (i) −x+1, −y+1, −z+2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2O···O15ii | 0.75 (3) | 1.98 (3) | 2.722 (2) | 167 (3) |
| O3—H3P···O18 | 0.83 (3) | 2.07 (3) | 2.892 (2) | 173 (3) |
| O4—H4O···O17iii | 0.74 (3) | 1.97 (3) | 2.710 (2) | 177 (3) |
| O5—H5O···O19 | 0.85 (3) | 1.82 (3) | 2.659 (2) | 169 (3) |
| O5—H5P···O9iv | 0.76 (3) | 2.10 (3) | 2.842 (2) | 164 (3) |
| O6—H6O···O7iv | 0.82 (3) | 1.95 (3) | 2.771 (2) | 173 (3) |
| O6—H6P···O11iii | 0.78 (2) | 2.00 (2) | 2.769 (2) | 170 (3) |
Symmetry codes: (ii) x+1/2, −y+1/2, z−1/2; (iii) −x+1/2, y−1/2, −z+3/2; (iv) x−1/2, −y+3/2, z−1/2.
Funding Statement
The authors are grateful for support from the Scientific Grant Agency of the Ministry of Education of Slovak Republic and Slovak Academy of Sciences VEGA, Project No. 1/0669/22.
References
- Amanchi, S. R. & Das, S. K. (2018). Front. Chem., 6, Article No. 469. [DOI] [PMC free article] [PubMed]
- Brandenburg, K. & Putz, H. (2005). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (2019). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2021). Instrument Service. Bruker AXS Inc., Madison, Wisconsin, USA.
- Gorzsás, A., Andersson, I. & Pettersson, L. (2009). J. Inorg. Biochem. 103, 517–526. [DOI] [PubMed]
- Graia, M., Ksiksi, R. & Driss, A. (2008). J. Chem. Crystallogr. 38, 855–859.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Huang, X., Gu, X., Zhang, H., Shen, G., Gong, S., Yang, B., Wang, Y. & Chen, Y. (2021). J. CO2 Util. 45, Article No. 101419.
- Jin, K. P., Jiang, H. J., Wang, Y., Zhang, D. P., Mei, J. & Cui, S. H. (2018). J. Clust Sci. 29, 785–792.
- Klištincová, L., Rakovský, E. & Schwendt, P. (2010). Transition Met. Chem. 35, 229–236.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Luo, X., Li, F., Xu, B., Sun, Z. & Xu, L. (2012). J. Mater. Chem. 22, 15050–15055.
- Nohra, B., El Moll, H., Rodriguez Albelo, L. M., Mialane, P., Marrot, J., Mellot-Draznieks, C., O’Keeffe, M., Ngo Biboum, R., Lemaire, J., Keita, B., Nadjo, L. & Dolbecq, A. (2011). J. Am. Chem. Soc. 133, 13363–13374. [DOI] [PubMed]
- Pang, H., Meng, X., Ma, H., Liu, B. & Li, S. (2012). Z. Naturforsch. Teil B, 67, 855–859.
- Pettersson, L., Hedman, B., Nenner, A. M. & Andersson, I. (1985). Acta Chem. Scand. A, 39, 499–506.
- Rakovský, E. & Krivosudský, L. (2014). Acta Cryst. E70, m225–m226. [DOI] [PMC free article] [PubMed]
- Rehder, D. (2015). J. Inorg. Biochem. 147, 25–31. [DOI] [PubMed]
- Schmidt, H., Andersson, I., Rehder, D. & Pettersson, L. (2001). Chem. Eur. J. 7, 251–257. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Slebodnick, C. & Pecoraro, V. L. (1998). Inorg. Chim. Acta, 283, 37–43.
- Udomvech, A., Kongrat, P., Pakawatchai, C. & Phetmung, H. (2012). Inorg. Chem. Commun. 17, 132–136.
- Wang, L., Sun, X. P., Liu, M. L., Gao, Y. Q., Gu, W. & Liu, X. (2008). J. Clust Sci. 19, 531–542.
- Wang, X., Sun, J., Lin, H., Chang, Z. & Liu, G. (2016). Inorg. Chem. Commun. 73, 152–156.
- Xu, W., Jiang, F., Zhou, Y., Xiong, K., Chen, L., Yang, M., Feng, R. & Hong, M. (2012). Dalton Trans. 41, 7737–7745. [DOI] [PubMed]
- Yerra, S. & Das, S. K. (2017). J. Mol. Struct. 1146, 23–31.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022003449/yz2017sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022003449/yz2017Isup2.hkl
CCDC reference: 2156016
Additional supporting information: crystallographic information; 3D view; checkCIF report




