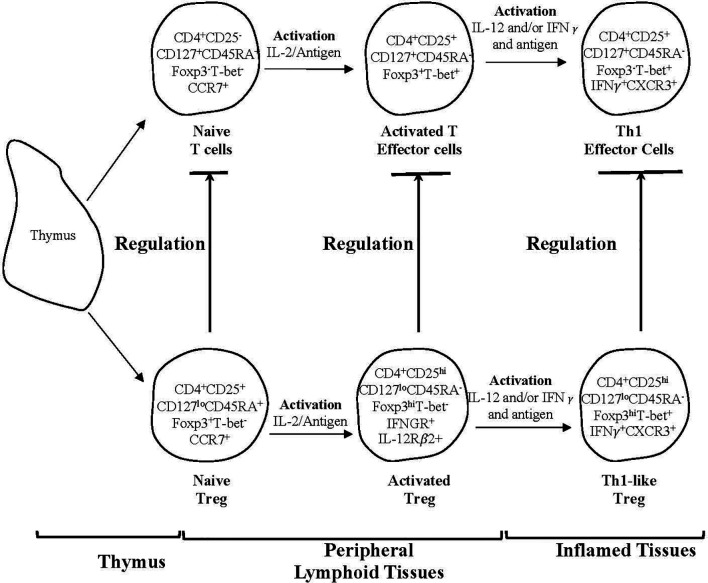
Figure 3.
A schematic representation of two subpopulations of CD4+T cells produced by the thymus and one of several pathways for their activation by an antigen and cytokines in peripheral lymphoid tissues and sites of inflammation. The activation by an antigen of effector lineage CD4+CD25-CD127+CD45RA+Foxp3- cells induces them to produce cytokines that promotes activation of CD25+CD127loCD45RA+Foxp3+Treg that have been activated by antigen. This figure shows the parallel pathways of activation of effector and regulatory CD4+T cells, when producing and being activated by Type-1 cytokines. The cytokines produced by the effector cells are required for the full activation of Treg. Both lineages of cells have been produced by thymus and have migrated to peripheral lymphoid tissue. Their subsequently recirculation from lymphoid tissue to blood and back to lymphoid tissue, is promoted by expression of CD62L and CCR7. This recirculation increases their chances of recognizing antigens. In peripheral lymphoid tissue upon recognition of an antigen, both effector and regulatory CD4+T cell populations are activated and proliferate. Effector lineage CD4+T cells start producing IL-2 and express IL-2R including CD25 (IL-2Ra chain). Naïve resting Treg expand polyclonally. During an immune response naïve/resting CD4+CD25+CD127loCD45RA+Foxp3+T-bet-CCR7+Treg are activated by an antigen and the IL-2 produced by activated T effector cells and are induced to express the receptor for late Th1 cytokines IL-12 and IFN−γ. Naïve CD4+CD25+CD127+CD45RA+Foxp3-T-bet-CCR7+T cells also acquire CD25, Foxp3 and T-bet expression but no longer express CD45RA. Transient expression of Foxp3 and CD25 on activated effector T cells blurs the distinction between Treg and effector T cells. In the event of ongoing immune response, activated T effector cells, in the presence of IL-2 and IFN-γ get further activated to express the transcription factor t-bet and the chemokine receptor CXCR3. These activated effector CD4+T cells produce IFN-γ, which together with IL-12 further activate Treg to Th1-like Treg (CD4+CD25hiCD127loCD45RA-Foxp3hiT-bet+IFN-γ+ CXCR3+). Th1-like Treg express mRNA for Th1 transcription factor T-bet, Th1 cytokine IFN-γ and Th1 chemokine receptor CXCR3. Expression of CXCR3 enables these Treg to migrate to inflamed tissues, where they control immune inflammation as in the graft and promote tolerance. Th-like Treg, such as Th1-like Treg are the mediators of transplant tolerance and are a hundred to a thousand-fold more potent at suppression of rejection than naïve resting Treg. This figure only represents one pathway of activation of Treg and there are others such as Th-2 like Treg promoted by Th2 cells and Type-2 cytokines. The survival of highly activated Treg is dependent on continued antigen stimulation and key cytokines produced by the inflammatory response, IL-2 alone does not sustain these cells and may inhibit them.