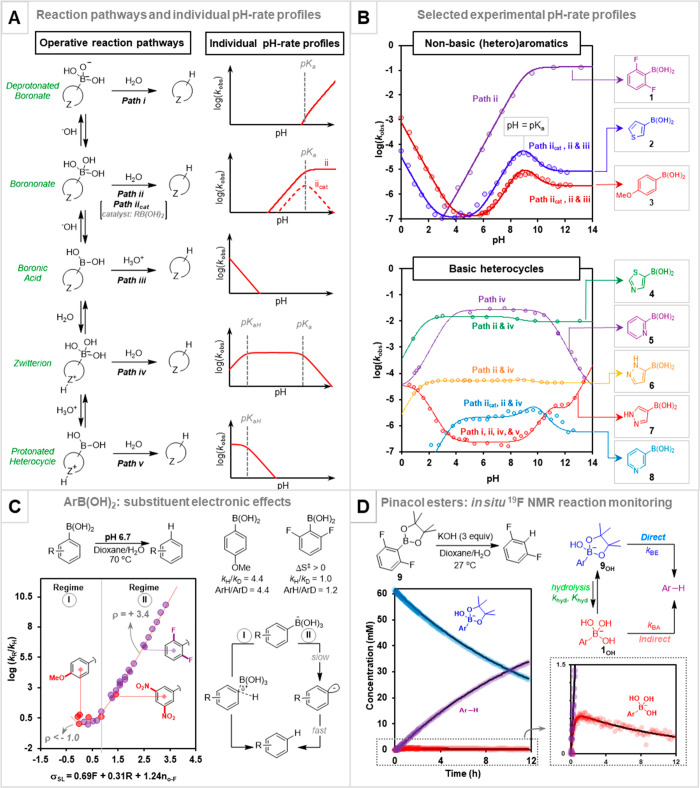
Figure 1.
(A) Overarching kinetic model (pathways i–v) for pH-mediated speciation and protodeboronation. (B) Example pH–rate profiles for the protodeboronation of selected (hetero)arylboronic acids (50 mM, 50% vol. aq. dioxane, 70 °C). (C) Modified Swain–Lupton analysis of the protodeboronation of arylboronic acids. (D) Competing direct and indirect protodeboronation of boronate esters. Data from refs (1), (2), and (30).