Figure Legend: Proposed mechanisms of anti-tumor activity for ONC201 and potential biomarkers for future study.
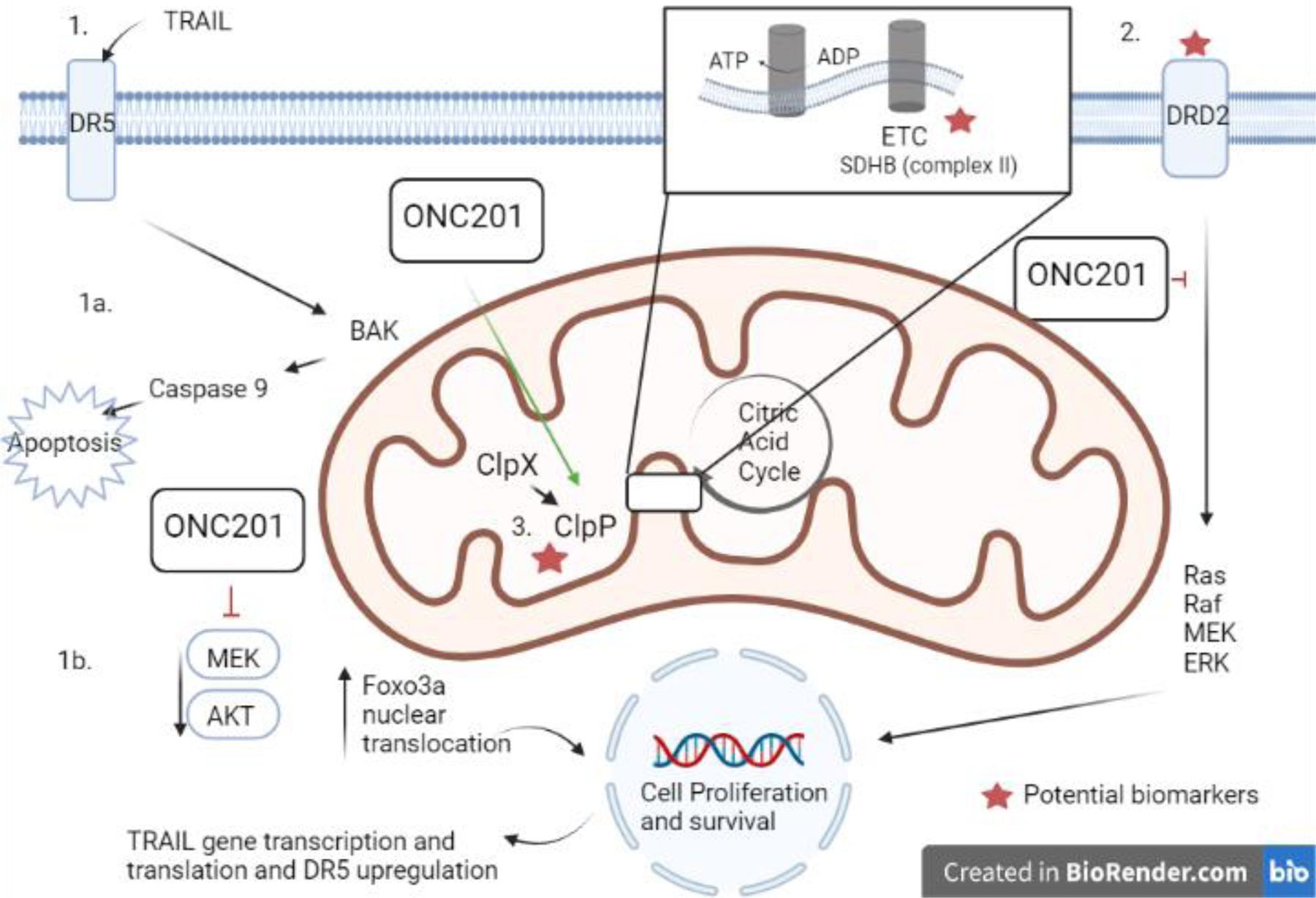
ONC201 is an orally administered imipridone that has demonstrated anti-tumor activity in a number of preclinical models and was found to have clinical efficacy in patients with pheochromocytoma and paraganglioma as reported by Anderson and colleagues in this issue of CCR. (1) Studies utilizing predominantly colon cancer preclinical models identified that ONC201 (originally named TRAIL-inducing compound 10, or TIC 10) induced TRAIL leading to apoptosis (1a) by downregulation of AKT and ERK, and that this anti-tumor activity was dependent on that transcriptional factor Foxo3a which leads to upregulation of TRAIL death receptor 5 (DR5) (1b, Ref 1). (2) ONC201 was also found to be an antagonist of the dopamine D2 receptors (DRD2), a G protein-coupled receptor that acts through ERK and Akt signaling to promotes cancer cell survival. (3) Lastly, ONC201 is also a direct activator of caseinolytic protease P (ClpP), a serine protease located in the mitochondrial matrix that regulates oxidative phosphorylation typically dependent on chaperone protein CLpX. This dependence is disrupted by ONC201 binding, leading to ClpP activation resulting in mitochondrial proteolysis including destruction of components of the electron transport chain. Given the role of succinate dehydrogenase as a bridge between the TCA and the electron transport chain, possibility of tumor suppressor SDH mutations as a potential biomarker for ONC201 could be explored in preclinical models as well as in future clinical studies.
ETC – electron transport chain; DRD2 - dopamine D2 receptor; DR5- death receptor 5; TRAIL - TNF-related apoptosis-inducing ligand
