Central Illustration. Deep phenotyping in the next era of pulmonary hypertension.
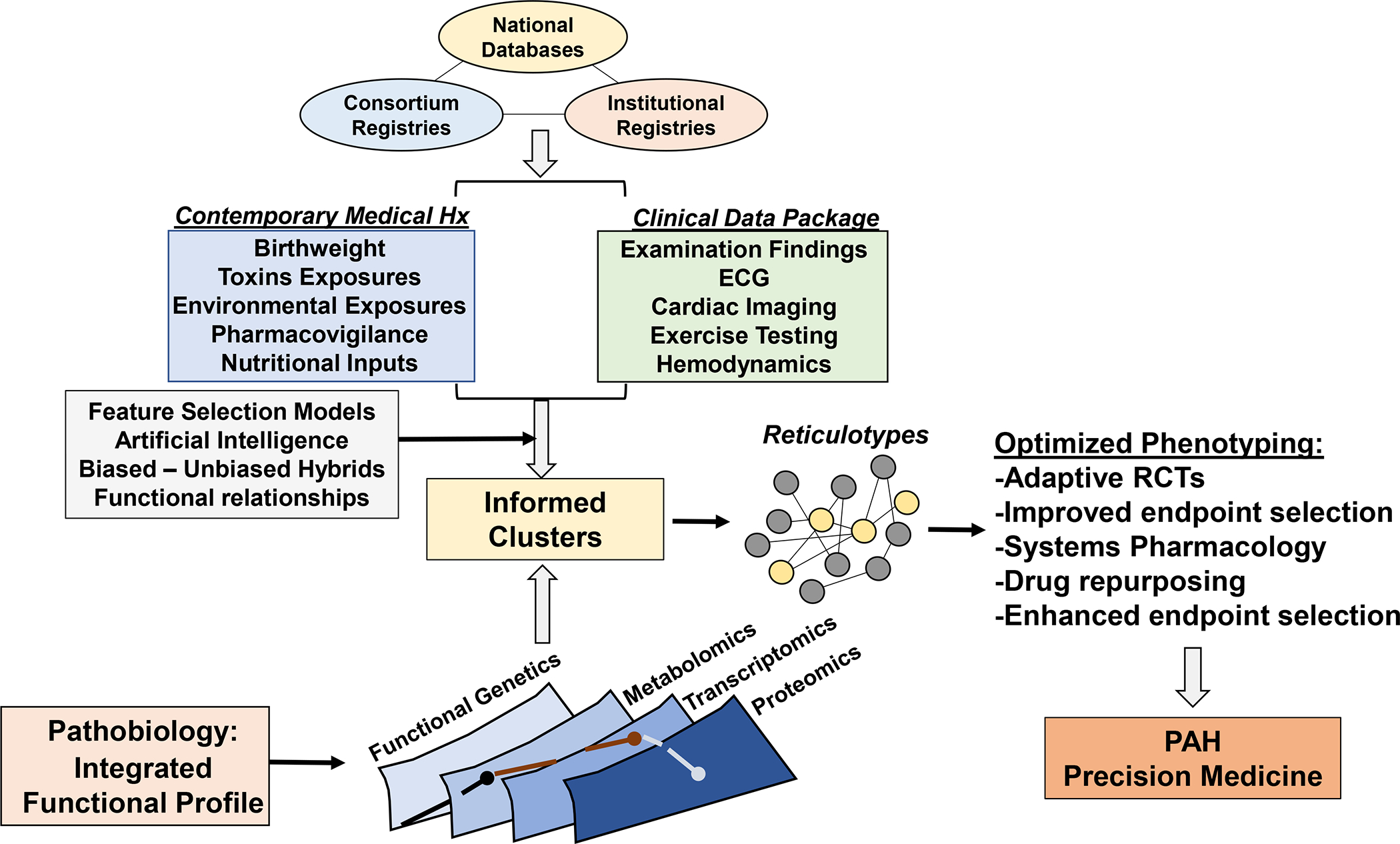
A substantiation number of large registries through foundation support, international collaborations, and institutional registries collecting clinical and biological data have emerged over the past 10 years. Integration of these data, coupled with unbiased and biased analytical methodologies, will be critical for clarifying the phenotypic profile of pulmonary hypertension. Expanding the range of collected data to non-conventional parameters, including perinatal events, nutritional patterns, toxic exposures, and pharmacovigilance programs (as already reported in France) via electronic medical record-based systems and other personalized device mechanisms will be critical for integrating genetic risk with acquired disease determinants. Ultimately, phenotyping patients will hinge on the identification of functionally important molecular pathways, inclusive of various –omics data, to generate reticulotypes. In turn, valid reticulocytes are well-positioned to optimize clinical trial design and establish a path toward individualized drug selection and, hence, precision medicine.
