Figure 7.
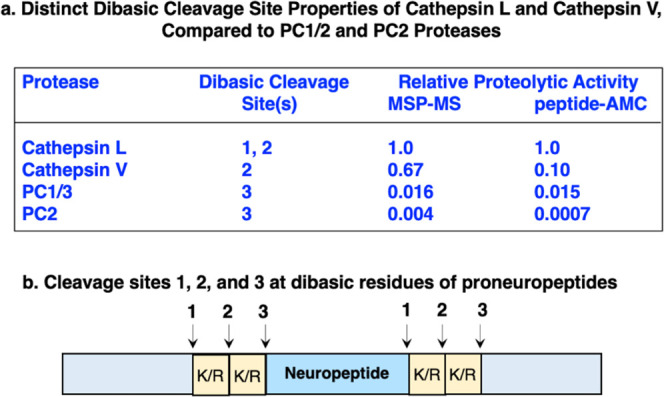
Distinct dibasic cleavage properties of cathepsin L and cathepsin V cysteine proteases compared to PC1/3 and PC2 serine proteases involved in proneuropeptide processing. (a) Information is shown for the relative proteolytic activity by the MSP-MS analyses of peptide library substrates and relative proteolytic activity observed in peptide-AMC assays using standard substrates for cathepsin L and cathepsin V (Z-F-R-AMC), and PC1/3 and PC2 (pERTKR-AMC). (b) Locations of dibasic cleavage sites (#1, 2, and 3) for each of the proneuropeptide processing proteases cathepsin L, cathepsin V, PC1/3, and PC2 are indicated.
