Abstract
OBJECTIVES
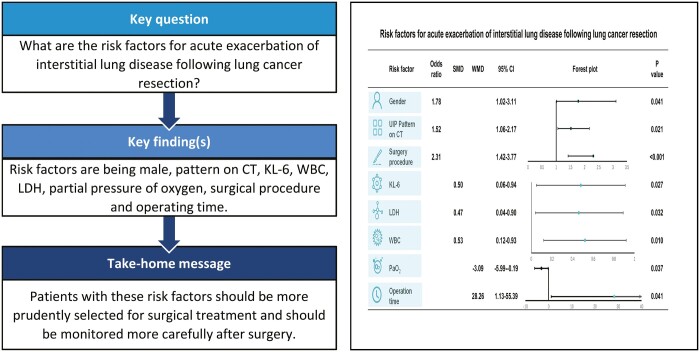
The aim of this study was to investigate the risk factors for acute exacerbation (AE) of interstitial lung disease (ILD) following lung cancer resection.
METHODS
We performed a literature screening on the databases including PubMed, Embase, Ovid MEDLINE® and the Web of Science for related studies published up to January 2021. Eligible studies were included and data on risk factors related to postoperative AE were extracted. All analyses were performed with random-effect model.
RESULTS
A total of 12 studies of 2655 lung cancer patients with ILD were included in this article. The meta-analysis indicated that male [odds ratios (ORs) = 1.78, 95% confidence interval (CI): 1.02–3.11, P = 0.041], usually interstitial pneumonia pattern on CT (OR = 1.52, 95% CI: 1.06–2.17, P = 0.021), Krebs von den Lungen-6 [standardized mean difference (SMD) = 0.50, 95% CI: 0.06–0.94, P = 0.027], white blood cell (SMD = 0.53, 95% CI: 0.12–0.93, P = 0.010), lactate dehydrogenase (SMD = 0.47, 95% CI: 0.04–0.90, P = 0.032), partial pressure of oxygen (weighted mean difference = −3.09, 95% CI: −5.99 to −0.19, P = 0.037), surgery procedure (OR = 2.31, 95% CI: 1.42–3.77, P < 0.001) and operation time (weighted mean difference = 28.26, 95% CI: 1.13–55.39, P = 0.041) were risk factors for AE of ILD following lung cancer resection.
CONCLUSIONS
We found that males, usually interstitial pneumonia pattern on CT, higher levels of Krebs von den Lungen-6, lactate dehydrogenase, white blood cell, lower partial pressure of oxygen, greater scope of operation and longer operation time were risk factors for AE of ILD following lung cancer resection. Patients with these risk factors should be more prudently selected for surgical treatment and be monitored more carefully after surgery.
Keywords: Interstitial lung disease, Lung cancer surgery, Acute exacerbation, Postoperative complications, Risk factors
Interstitial lung disease (ILD), also known as diffuse parenchymal lung disease, is characterized by progressive dyspnoea and irreversible deterioration of lung function [1, 2].
INTRODUCTION
Interstitial lung disease (ILD), also known as diffuse parenchymal lung disease, is characterized by progressive dyspnoea and irreversible deterioration of lung function [1, 2]. It is already known that ILD shares similar risk factors and pathophysiological processes with lung cancer [3, 4], and there have been studies confirming that the relative risk of lung cancer in patients with ILD is 3.5–7.8 times that of the general population [5–9].
Surgery is recommended by the guidelines as one of the treatment strategies for lung cancer patients with ILD [10]. However, it may cause acute exacerbation (AE) of ILD after surgery, which often requires more intensive care and may cause serious consequences. A multicentre retrospective study [11] found that the incidence of postoperative AE of ILD in lung cancer patients with ILD was 9.3% [95% confidence interval (CI): 8.0–10.8]. In other single-institution studies, the incidence may be as high as 32.1% [12]. Although the postoperative 30-day mortality rate of lung cancer patients has gradually decreased in recent years [13], it was 43.9% among the population with AE of ILD [11], which was the leading course of death after lung cancer surgery [14]. Therefore, exploring the risk factors for AE of ILD following lung cancer resection is of great significance for surgeons to weigh the pros and cons of surgery and prevent postoperative AE.
Although existing studies have found some risk factors for AE of ILD following lung cancer resection, such as surgical methods, lung function, gender and tumour location, the conclusions were inconsistent and the number of included patients was limited [11, 15–17]. The purpose of this systematic review and meta-analysis is to explore the risk factors for AE of ILD following lung cancer resection.
METHODS
Search strategy
This study was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
According to the guidelines for performing meta-analyses, we performed a comprehensive literature screening on the acknowledged databases including PubMed, Embase, Ovid MEDLINE® and the Web of Science for related studies published up to January 2021. Search strategy was comprised of ‘lung cancer’, ‘surgery’, ‘interstitial lung disease’, ‘interstitial pneumonia’, ‘interstitial lung disease’. The following search items were used in PubMed: ((((interstitial lung disease [Title/Abstract]) OR (interstitial pneumonia [Title/Abstract])) OR (interstitial lung disease [Title/Abstract])) AND (lung cancer [Title/Abstract])) AND (surgery).
Inclusion and exclusion criteria
We included studies that fulfilled the following criteria: (i) all included adult patients were diagnosed with lung cancer by pathology and ILD by computed tomography or pathology; (ii) all included adult patients were treated with surgery; (iii) studies including risk factors of AE of ILD following lung cancer resection; and (iv) full-text publication in English.
Studies were excluded if they met one or more of the following criteria: (i) case report, letter, experimental studies, review, conference abstract and introduction; (ii) comparable data could not be extracted; and (iii) basic essential data were incomplete.
Data abstraction and outcome measures
Two experienced researchers (Jianqi Hao and Cong Chen) independently analysed finally defined articles for primary parameters, which indicated the risk factor of ILD and secondary parameters concerning the basic information of the article. The basic information data abstracted from eligible studies included the year of publication and first author's name, country, number of patients, type of studies and so on. The related risk factor parameters, including gender, age, cancer stage, tumour location, surgery procedure, lung functions and laboratory examination, were abstracted into a standard data table.
QUALITY ASSESSMENT AND PUBLISH BIAS
The Newcastle–Ottawa Scale was used to assess the quality of original non-randomized studies by 2 researchers (Jianqi Hao and Cong Chen). The scale includes 3 aspects of evaluation: selection, comparability and exposure. The high-quality studies were defined as with at least 8 stars. Studies with at least 6 stars were included in our meta-analysis. If there were any discrepancies, they would be solved by discussion or consultation with a 3rd reviewer (Xiaohu Hao). To assess publication bias, funnel plots were generated for each result.
Statistical analysis
RevMan 5.3 software (freeware available from The Cochrane Collaboration, http://www.ccims.net/revman/download) and STATA version 16.0 (Stata Corp, College Station, TX, USA) were used to perform all the statistical data analysis. Weighted mean difference (WMD) or standardized mean difference (SMD) and 95% CI were used for continuous variables. Comparative odds ratios (ORs) were reported with their associated 95% CI for the dichotomous variable. The I2-statistic was used to assess the extent of heterogeneity of the included studies. All analyses were performed with random-effect model. A funnel plot was generated for each result. A value of P < 0.050 was considered statistically significant.
RESULTS
Included studies and characteristics
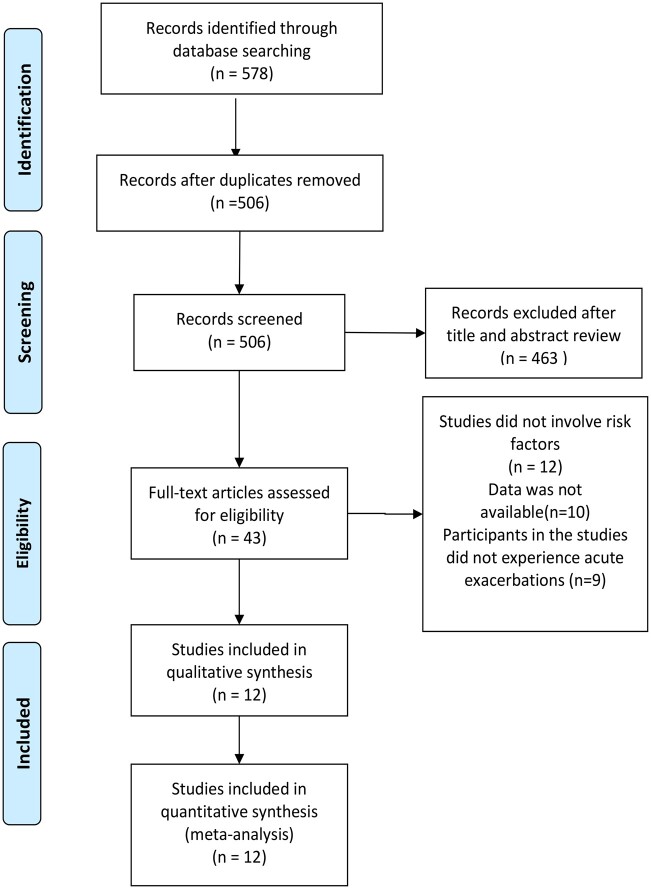
After a comprehensive literature screening, database searches retrieved 578 results, with 43 full-text articles assessed for eligibility. Twelve were in conformity with the inclusion criteria and included in our analysis (Fig. 1).
Figure 1:
Flow chart of the identification of relevant studies.
The basic information is shown in Table 1. The included studies were published from 2004 to 2020. A total of 2655 lung cancer patients with ILD were included in our analysis. ILD was mainly diagnosed by CT and pathology. Finally, we focused on the risk factors that may be associated with AE of ILD after lung cancer surgery.
Table 1:
Characteristics of the included studies
| Author | Year | Country | Sample size | No. acute exacerbation, n (%) | Recruitment period | Diagnosis of interstitial lung disease | Quality assessment |
|---|---|---|---|---|---|---|---|
| Fukui et al. [15] | 2020 | Japan | 337 | 14 (4.2) | 2009–2018 | CT | 8 |
| Iyoda et al. [18] | 2011 | Japan | 22 | 5 (22.7) | NA | Prior patient history, CT, Pathology | 7 |
| Kanzaki et al. [19] | 2011 | Japan | 40 | 12 (30) | 2001–2009 | CT, Pathology | 8 |
| Kobayashi et al. [16] | 2016 | Japan | 137 | 17 (12.4) | 2006–2015 | CT, Pathology | 8 |
| Koizumi et al. [20] | 2004 | Japan | 47 | 7 (14.9) | 1982–2003 | CT | 8 |
| Maniwa et al. [21] | 2013 | Japan | 89 | 8 (9.0) | 2002–2011 | CT | 8 |
| Oishi et al. [22] | 2020 | Japan | 31 | 5 (16.1) | 2012–2017 | CT | 8 |
| Sato et al. [11] | 2013 | Japan | 1763 | 164 (9.3) | 2000–2009 | Prior patient history, CT, Pathology | 8 |
| Shintani et al. [23] | 2010 | Japan | 40 | 6 (15.0) | 1990–2005 | Pathology | 8 |
| Suzuki et al. [12] | 2011 | Japan | 28 | 9 (32.1) | 2000–2006 | CT, Pathology | 8 |
| Taniguchi et al. [24] | 2017 | Japan | 59 | 5 (8.5) | 1994–2013 | Prior patient history, CT | 8 |
| Yano et al. [17] | 2012 | Japan | 62 | 6 (9.7) | 2004–2009 | HRCT | 8 |
NA: not available; HRCT: high-resolution computed tomography.
Positive risk factors for acute exacerbation of interstitial lung disease
Gender
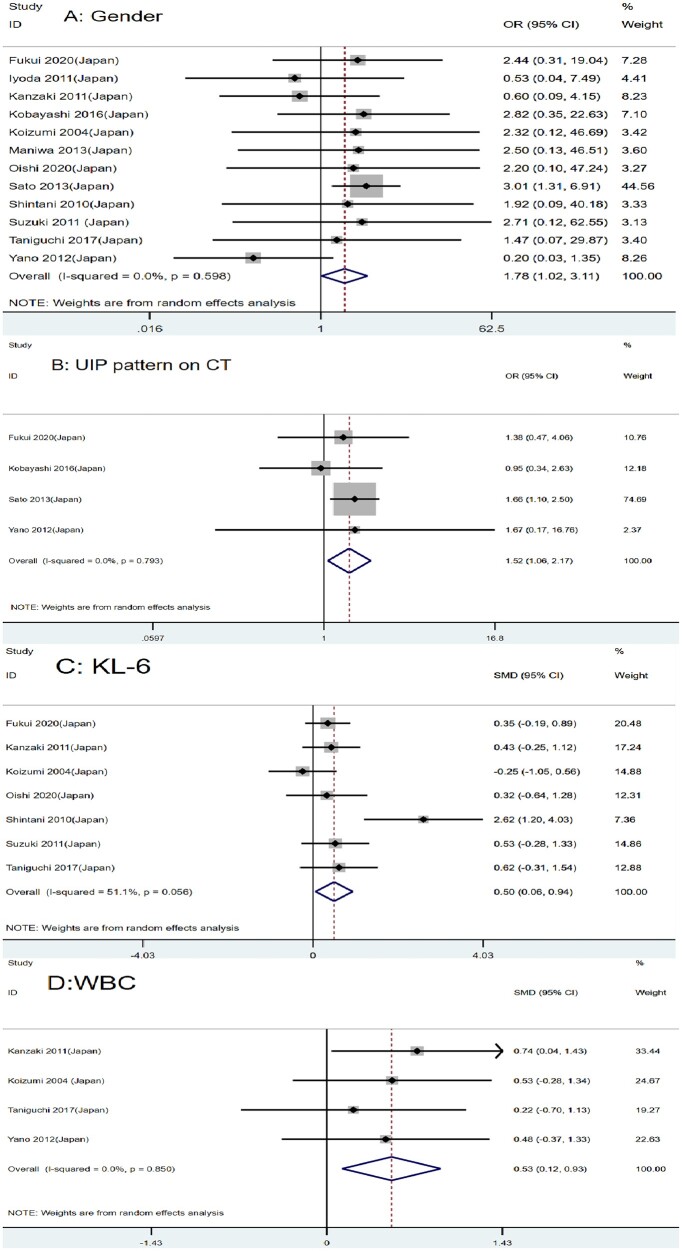
Twelve studies with 2655 patients reported the association between gender and AE of ILD after lung cancer surgery [11, 12, 15–24]. The result showed that male lung cancer patients with ILD may be more prone to suffer from AE of ILD than females (OR = 1.78, 95% CI: 1.02–3.11, P = 0.041) (Fig. 2A).
Figure 2:
Forest plot of the potential risk factors of acute exacerbation of interstitial lung disease following lung cancer resection: (A) gender; (B) usually interstitial pneumonia pattern on CT; (C) Krebs von den Lungen-6; and (D) white blood cell.
Usually interstitial pneumonia pattern on CT
Four articles involving 2299 patients reported the high risk of AE of ILD among lung cancer patients with usually interstitial pneumonia (UIP), which corresponded with our combined analysis (OR = 1.52, 95% CI: 1.06–2.17, P = 0.021) (Fig. 2B) [11, 15–17].
Laboratory examination
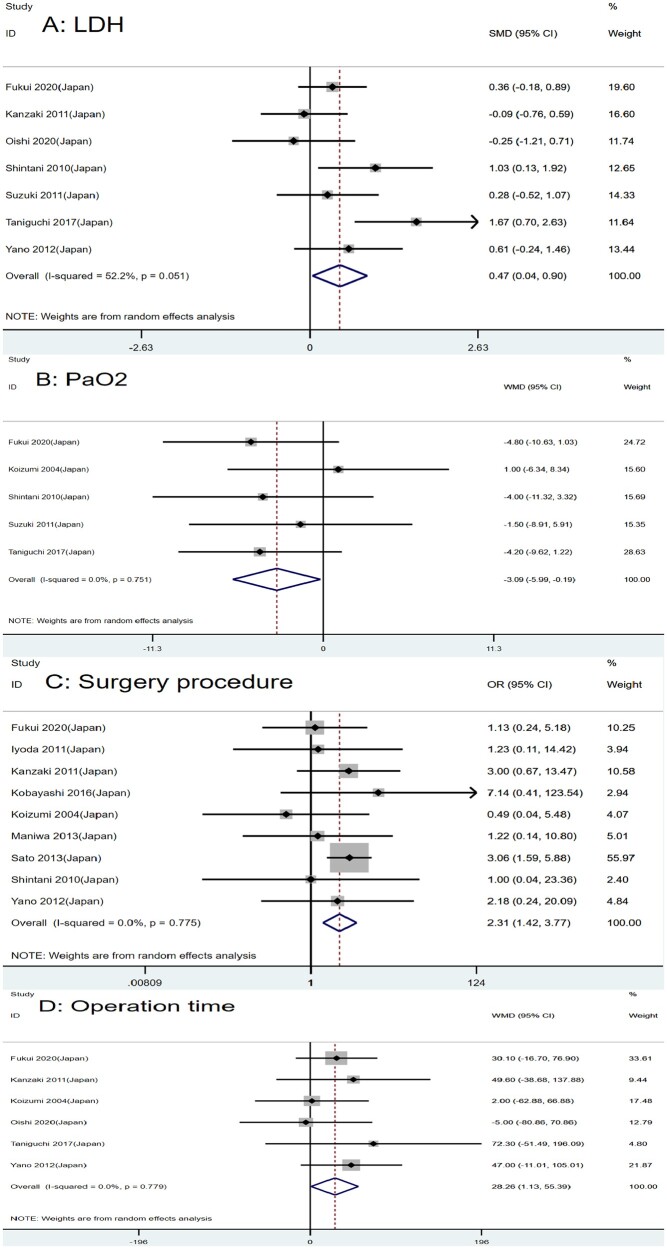
We also studied the role of laboratory examination in the postoperative AE of lung cancer patients with ILD. After a combined analysis of 568, 587 and 208 patients, respectively, we found that serum Krebs von den Lungen-6 (KL-6), white blood cell (WBC) and lactate dehydrogenase (LDH) might be potential risk factors for AE of ILD following lung cancer surgery (SMD = 0.50, 95% CI: 0.06–0.94, P = 0.027; SMD = 0.53, 95% CI: 0.12–0.93, P = 0.010; SMD = 0.47, 95% CI: 0.04–0.90, P = 0.032, respectively) (Figs 2C and D and 3A). A high level of those markers generally predicts a high risk of AE after surgery. C-reactive protein may not be a risk factor (WMD = 0.61, 95% CI: −0.32 to 1.55, P = 0.200) (Supplementary Material, Fig. S1A).
Blood gas analysis
Many researchers thought that blood gas analysis might be an effective predictor for AE of ILD after lung cancer surgery. However, according to our combined analysis of 5 studies with 511 patients [12, 15, 20, 23, 24], only partial pressure of oxygen was a risk factor for AE, while partial pressure of carbon dioxide was not (WMD = −3.09, 95% CI: −5.99 to −0.19, P = 0.037; WMD = 0.13, 95% CI: −1.25 to 1.51, P = 0.854, respectively) (Fig. 3B and Supplementary Material, Fig. S1B).
Figure 3:
Forest plot of the potential risk factors of acute exacerbation of interstitial lung disease following lung cancer resection: (A) lactate dehydrogenase; (B) partial pressure of oxygen; (C) surgery procedure; and (D) operation time.
Surgery procedure
Nine articles with 2527 patients studied the association between surgery procedure and AE of ILD after lung cancer surgery [11, 15–21, 23]. Our result suggested that patients undergoing sublobar resection were less likely to have AE of ILD. The greater the scope of surgery, the higher the incidence of acute postoperative exacerbations (OR = 2.31, 95% CI: 1.42–3.77, P < 0.001) (Fig. 3C).
Time of operation
Operation time is an essential factor affecting postoperative complications. Six studies with 576 patients reported the relationship between the operation time and AE of ILD following lung cancer resection [15, 17, 19, 20, 22, 24]. We found that operation time was a risk factor for AE (WMD = 28.26, 95% CI: 1.13–55.39, P = 0.041) (Fig. 3D).
Negative risk factors for acute exacerbation of interstitial lung disease
Pulmonary function
We also studied the relationship between pulmonary function and AE after surgery. The result showed that percentage of vital capacity (MD = −7.70, 95% CI: −16.60 to 1.21, P = 0.090), forced expiratory volume in 1 s (SMD = −0.01, 95% CI: −0.33 to 0.31, P = 0.955), % of forced expiratory volume in 1 s (WMD = 0.33 95% CI: −3.74 to 4.41, P = 0.873) and % of diffusion capacity for carbon monoxide (WMD = −3.16, 95% CI: −12.5 to 6.17, P = 0.506) might not be risk factors for AE of ILD.(Supplementary Material, Figs S1C and D and S2A and B).
Tumour location
We initially suspected that different tumour locations might affect the prognosis of patients, so we extracted the data of tumour location from 4 related studies with 248 patients [18, 20, 21, 24]. The result showed no significant difference in the incidence of AE of ILD following lung cancer resection whether the tumour was in the left or right lobe (OR = 1.29, 95% CI: 0.51–3.24, P = 0.594) (Supplementary Material, Fig. S2C).
The stage for lung cancer
Six articles with 507 patients studied the relationship between pathological cancer staging and AE [15, 17, 19–21, 23]. We found that the pathological stage was not the risk factor for postoperative AE (OR = 0.71, 95% CI: 0.38–1.35, P = 0.296) (Supplementary Material, Fig. S2D).
Quality assessment and publication bias
Standard quality evaluation of the 12 included studies was performed based on the Newcastle–Ottawa Scale. According to the evaluating system, the 12 included studies were reliable (Table 2). The funnel plot indicated that there was no publication bias in these studies (Supplementary Material, Fig. S3).
Table 2:
Result of the quality assessment by the Newcastle–Ottawa Scale
| Study | Selection |
Comparability | Outcome |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case definition | Representativeness of the cases | Selection of controls | Definition of controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non- response rate | |||
| Fukui et al. [15] | * | * | * | * | * | * | * | * | 8 |
| Iyoda et al. [18] | * | * | * | * | – | * | * | * | 7 |
| Kanzaki et al. [19] | * | * | * | * | * | * | * | * | 8 |
| Kobayashi et al. [16] | * | * | * | * | * | * | * | * | 8 |
| Koizumi [20] | * | * | * | * | * | * | * | * | 8 |
| Maniwa et al. [21] | * | * | * | * | * | * | * | * | 8 |
| Oishi et al. [22] | * | * | * | * | * | * | * | * | 8 |
| Sato et al. [11] | * | * | * | * | * | * | * | * | 8 |
| Shintani et al. [23] | * | * | * | * | * | * | * | * | 8 |
| Suzuki et al. [12] | * | * | * | * | * | * | * | * | 8 |
| Taniguchi et al. [24] | * | * | * | * | * | * | * | * | 8 |
| Yano et al. [17] | * | * | * | * | * | * | * | * | 8 |
*This criterion is met.
DISCUSSION
Postoperative AE of ILD was defined as those that occur within 30 days after surgery and cannot be explained by lung infections or other diseases, progressive dyspnoea, increasing interstitial shadows on chest CT or X-ray, and decreased blood oxygen partial pressure by >10 mmHg [10, 25]. The pulmonary function of patients with ILD and lung cancer is often significantly impaired, and surgery is likely to be a fatal blow to induce the AE of ILD. However, there is no recognized predictor, which is an urgent problem for surgeons to identify patients prone to AE of ILD following lung cancer surgery. As far as we know, this is the first meta-analysis to explore risk factors of AE of ILD following lung cancer surgery. In this study, 8 factors were found to be the potential risk factors for AE of ILD following lung cancer surgery: gender, UIP pattern on CT, KL-6, LDH, WBC, partial pressure of oxygen, surgery procedure and operation time.
Laboratory examination, chest radiography and spirometry are the most important objective preoperative assessment of lung cancer patients. KL-6, a circulating glycoprotein secreted by alveolar epithelium and bronchial epithelium, is a crucial serum biomarker to assess the activity of interstitial pneumonia [26]. KL-6 can predict the survival outcomes of ILD patients without lung cancer and the early clinical effectiveness of therapy [27–29]. This study confirmed that KL-6 was a significant risk factor for postoperative AE. LDH is also a biomarker of interstitial pneumonia activity and can be used to predict postoperative AE [23, 30, 31]. However, the elevation of LDH is gradual and often occurs in the late stage of the disease, so it may not be suitable as an early predictive marker [32]. Other serum biomarkers such as surfactant proteins A and D may also have predictive effects [33], but they were not included in our analysis due to the small number of studies. We found that patients diagnosed with UIP based on preoperative chest CT were more likely to experience postoperative AE, possibly because the UIP group had more typical honeycomb lesions, which were associated with prognosis and fibrosis [2]. Suzuki et al. found that the scores for fibrosis, consolidation and ground-glass opacity were significantly higher in AE patients [12]. Therefore, the type and severity of lesions in patients should be carefully evaluated by chest computed tomography (CT) before surgery. Positron emission tomography (PET–CT) plays a significant role in the diagnosis and staging of lung cancer. Some studies have also found that maximum standardized uptake value and the ratio of the ILD area’s peak standard uptake value (SUV) to of the mediastinum’s mean SUV can predict postoperative AE [22, 34, 35]; more large-scale studies are necessary to confirm. It was a pity that pulmonary function examination could not predict postoperative AE in this study, which was consistent with previous studies [19, 36], though pulmonary function examination is an effective method to evaluate the severity of pulmonary lesions in patients. However, some studies have found that diffusing capacity of the lung for carbon monoxide and vital capacity percentage may have a predictive effect, but the conclusions were not consistent [11, 23, 37]. We think it may be due to the heterogeneity of lung function tests in different institutions and individual differences in patients.
Choosing an appropriate surgical procedure for lung cancer patients with ILD is a dilemma for surgeons to make serious decisions. This study found that the larger the scope of resection, the higher risk of AE, which may be caused by lymphatic drainage disorder and increased endothelium pressure after surgery. Wedge resection was less likely to trigger postoperative AE than segmentectomy and lobectomy [11]. However, limited excision may lead to poor oncology outcomes. Sato et al. [38] reported that patients with stage IA lung cancer who underwent wedge resection had poorer long-term survival than lobectomy and segmentectomy (OR 2.98 (95% CI, 1.56–5.68, P = 0.001) and 2.56 (95% CI, 1.15–5.67, P = 0.021), respectively). Tsutani et al. reached a different conclusion. There was no statistical difference in overall survival between patients who underwent lobectomy and sublobar resection with stage IA lung cancer and ILD (P = 0.87) [39]. The jury is still out on this, and more studies are needed to address this question. Surgeons need to comprehensively consider short-term and long-term prognoses and choose the most appropriate surgical procedure. Operative time is also a predictor of postoperative AE, but it may be related to the surgical procedure. Koizumi et al. [20] compared the effects of video-assisted thoracoscopic surgery (VATS) and thoracotomy on postoperative AE. Although VATS did not prevent AE, the incidence of postoperative complications in VATS seemed to be lower, and VATS may be one of the beneficial options for patients with lung cancer and ILD.
In addition, some studies have found that age, smoking history, pathological type, stage, single-lung ventilation, postoperative pyothorax and chest-tube drainage have predictive effects [11, 15, 16, 18, 20, 21]. However, these results were not included or confirmed in this study, thus remaining to be clarified by further studies. Staging is of great value in treatment decisions and prognosis prediction of cancer patients, but it does not seem to be a prognostic factor for AE. Only Iyoda et al.'s [18] study found that patients with advanced-stage were more likely to develop AE, but this study only included 5 patients with AE (P = 0.0344). We think it is due to the fact that patients with advanced lung cancer and ILD rarely undergoes surgical treatment. Some studies have speculated that the high concentration of oxygen used in single-lung ventilation during lung cancer surgery may lead to AE by producing reactive oxygen species [40–42]. There are too few studies to be included in the meta-analysis. More clinical trials are needed to determine whether AE can be avoided by reducing oxygen concentration.
The current guidelines do not recommend the use of drugs to prevent postoperative AE. Preoperative prophylactic corticosteroid and sivelestat seem to be ineffective [10, 43]. However, a small prospective trial found that pirfenidone may have a preventive effect [44]. The guidelines weakly recommend the use of corticosteroid therapy, including pulse therapy and immunosuppressive therapy [10, 25, 45, 46]. However, the level of evidence is low; there is still much to be done to improve treatment strategies.
Limitations
This study has some limitations. First, all the included studies were retrospective, so there may be some bias that cannot reflect the actual situation of all patients. Second, almost all the study population were from Japan, and the results may lack generality. It is hoped that large-scale and multicentre studies can be done to confirm the results of this article in the future.
CONCLUSION
We found that gender, UIP pattern on CT, KL-6, LDH, WBC, partial pressure of oxygen, surgery procedure and operation time were risk factors for AE of ILD following lung cancer resection. Patients with these risk factors should be more prudently selected for surgical treatment and be monitored more carefully after surgery.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENT
All authors approved the final manuscript as submitted and agreed to be accountable for all of the work.
Funding
This work is supported by 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD18021, ZYJC18009) (to Dr. Lunxu Liu), Post-Doctor Research Project, West China Hospital, Sichuan University (Grant 2020HXBH108) (to Dr. Jian Zhang), Postdoctoral Science Foundation (Grant 2021M692268) (to Dr. Jian Zhang).
Conflict of interest: none declared.
Data Availability Statement
All relevant data are within the manuscript and its supporting Information files.
Author contributions
Xiaohu Hao: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Writing—original draft. Jianqi Hao: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft. Cong Chen: Conceptualization; Data curation; Formal analysis; Writing—review & editing. Haoning Peng: Data curation; Writing—original draft. Jian Zhang: Formal analysis; Writing—review & editing. Qi Cao: Formal analysis; Writing—review & editing. Lunxu Liu: Conceptualization; Funding acquisition.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Nuria M Novoa, Rizwan A. Qureshi, Mohammad Behgam Shadmehr and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AE
Acute exacerbation
- CI
Confidence interval
- ILD
Interstitial lung disease
- KL-6
Krebs von den Lungen-6
- LDH
Lactate dehydrogenase
- ORs
Odds ratios
- SMD
Standardized mean difference
- UIP
Usually interstitial pneumonia
- WBC
White blood cell
- WMD
Weighted mean difference
REFERENCES
- 1.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG. et al. ; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hironaka M, Fukayama M.. Pulmonary fibrosis and lung carcinoma: a comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int 1999;49:1060–6. [DOI] [PubMed] [Google Scholar]
- 4. Richeldi L, Collard HR, Jones MG.. Idiopathic pulmonary fibrosis. Lancet (London, England) 2017;389:1941–52. [DOI] [PubMed] [Google Scholar]
- 5. Lee HY, Lee J, Lee C-H, Han K, Choi SM.. Risk of cancer incidence in patients with idiopathic pulmonary fibrosis: a nationwide cohort study. Respirology 2021;26(2):180–187. [DOI] [PubMed] [Google Scholar]
- 6. Naccache J-M, Gibiot Q, Monnet I, Antoine M, Wislez M, Chouaid C. et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829–44. +. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubbard R, Venn A, Lewis S, Britton J.. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5–8. [DOI] [PubMed] [Google Scholar]
- 8. Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R.. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 2007;101:2534–40. [DOI] [PubMed] [Google Scholar]
- 9. Mizuno S, Takiguchi Y, Fujikawa A, Motoori K, Tada Y, Kurosu K. et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology 2009;14:377–83. [DOI] [PubMed] [Google Scholar]
- 10. Homma S, Bando M, Azuma A, Sakamoto S, Sugino K, Ishii Y. et al. ; Ministry of Health, Labour and Welfare, the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Diseases, and Japanese Respiratory Society. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268–91. [DOI] [PubMed] [Google Scholar]
- 11. Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K. et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604–11.e3. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Yonemori Y. et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today 2011;41:914–21. [DOI] [PubMed] [Google Scholar]
- 13. Jakobsen E, Palshof T, Osterlind K, Pilegaard H.. Data from a national lung cancer registry contributes to improve outcome and quality of surgery: Danish results. Eur J Cardiothorac Surg 2009;35:348–52, discussion 52. [DOI] [PubMed] [Google Scholar]
- 14. Masuda M, Kuwano H, Okumura M, Amano J, Arai H, Endo S. et al. ; Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukui M, Takamochi K, Ando K, Matsunaga T, Hattori A, Oh S. et al. Advantages and disadvantages of corticosteroid use for acute exacerbation of interstitial pneumonia after pulmonary resection. Gen Thorac Cardiovasc Surg 2021;69:472–7. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi S, Karube Y, Nishihira M, Inoue T, Araki O, Maeda S. et al. Postoperative pyothorax a risk factor for acute exacerbation of idiopathic interstitial pneumonia following lung cancer resection. Gen Thorac Cardiovasc Surg 2016;64:476–80. [DOI] [PubMed] [Google Scholar]
- 17. Yano M, Sasaki H, Moriyama S, Hikosaka Y, Yokota K, Kobayashi S. et al. Postoperative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact CardioVasc Thorac Surg 2012;14:146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyoda A, Jiang S-X, Amano H, Ogawa F, Matui Y, Kurouzu N. et al. Prediction of postoperative exacerbation of interstitial pneumonia in patients with lung cancer and interstitial lung disease. Exp Ther Med 2011;2:1073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanzaki M, Kikkawa T, Maeda H, Kondo M, Isaka T, Shimizu T. et al. Acute exacerbation of idiopathic interstitial pneumonias after surgical resection of lung cancer. Interact CardioVasc Thorac Surg 2011;13:16–20. [DOI] [PubMed] [Google Scholar]
- 20. Koizumi K, Hirata T, Hirai K, Mikami I, Okada D, Yamagishi S. et al. Surgical treatment of lung cancer combined with interstitial pneumonia: the effect of surgical approach on postoperative acute exacerbation. Ann Thorac Cardiovasc Surg 2004;10:340–6. [PubMed] [Google Scholar]
- 21. Maniwa T, Isaka M, Nakagawa K, Ohde Y, Okumura T, Endo M. et al. Chest-tube drainage is a sign of acute exacerbation of interstitial lung disease associated with lung cancer. Surg Today 2013;43:408–11. [DOI] [PubMed] [Google Scholar]
- 22. Oishi H, Sakurada A, Notsuda H, Tanaka R, Takanami K, Saito R. et al. Correlation between preoperative (18)F-FDG PET/CT findings and postoperative short-term prognosis in lung cancer patients with idiopathic interstitial pneumonia after lung resection. Respir Investig 2021;59(1):106–113. [DOI] [PubMed] [Google Scholar]
- 23. Shintani Y, Ohta M, Iwasaki T, Ikeda N, Tomita E, Kawahara K. et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg 2010;58:182–5. [DOI] [PubMed] [Google Scholar]
- 24. Taniguchi D, Yamasaki N, Miyazaki T, Tsuchiya T, Matsumoto K, Hatachi G. et al. The surgical outcomes of lung cancer combined with interstitial pneumonia: a single-institution report. Surg Today 2017;47:1397–404. [DOI] [PubMed] [Google Scholar]
- 25. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK. et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M.. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68–73. [DOI] [PubMed] [Google Scholar]
- 27. Sakuma T, Takahashi K, Ohya N, Usuda K, Handa M.. Serum KL-6, a novel mucin-like glycoprotein, as an indicator of interstitial pneumonitis following lobectomy. Surg Today 1999;29:121–8. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama A, Kohno N, Hamada H, Sakatani M, Ueda E, Kondo K. et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:1680–4. [DOI] [PubMed] [Google Scholar]
- 29. Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S. et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;299:L3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Krugten M, Cobben NA, Lamers RJ, van Dieijen-Visser MP, Wagenaar SS, Wouters EF. et al. Serum LDH: a marker of disease activity and its response to therapy in idiopathic pulmonary fibrosis. Neth J Med 1996;48:220–3. [DOI] [PubMed] [Google Scholar]
- 31. Cabanillas Martín J, Peces-Barba G, Avilés Inglés MJ, Renedo Pascual G, González Mangado N, Vallejo Galbete J.. Serum LDH as a biochemical marker of activity in interstitial pneumopathy. Rev Clin Esp 1987;181:398. [PubMed] [Google Scholar]
- 32. Muraoka M, Tagawa T, Akamine S, Oka T, Tsuchiya T, Araki M. et al. Acute interstitial pneumonia following surgery for primary lung cancer. Eur J Cardiothorac Surg 2006;30:657–62. [DOI] [PubMed] [Google Scholar]
- 33. Ishii H, Mukae H, Kadota J, Kaida H, Nagata T, Abe K. et al. High serum concentrations of surfactant protein A in usual interstitial pneumonia compared with non-specific interstitial pneumonia. Thorax 2003;58:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maniwa T, Endo M, Isaka M, Nakagawa K, Ohde Y, Okumura T. et al. Acute exacerbation of interstitial lung disease with lung cancer after surgery: evaluation with 2-[18]-fluoro-2-deoxy-d-glucose positron emission tomography. Surg Today 2014;44:494–8. [DOI] [PubMed] [Google Scholar]
- 35. Yamamichi T, Shimada Y, Masuno R, Ohira T, Abe S, Yoshimura A. et al. Association between F-18 fluorodeoxyglucose uptake of noncancerous lung area and acute exacerbation of interstitial pneumonia in patients with lung cancer after resection. J Thorac Cardiovasc Surg 2020;159:1111–18. e2. [DOI] [PubMed] [Google Scholar]
- 36. Sugiura H, Takeda A, Hoshi T, Kawabata Y, Sayama K, Jinzaki M. et al. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg 2012;93:937–43. [DOI] [PubMed] [Google Scholar]
- 37. Park JS, Kim HK, Kim K, Kim J, Shim YM, Choi YS.. Prediction of acute pulmonary complications after resection of lung cancer in patients with preexisting interstitial lung disease. Thorac Cardiovasc Surg 2011;59:148–52. [DOI] [PubMed] [Google Scholar]
- 38. Sato T, Watanabe A, Kondo H, Kanzaki M, Okubo K, Yokoi K. et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64–9.70.e1–2. [DOI] [PubMed] [Google Scholar]
- 39. Tsutani Y, Mimura T, Kai Y, Ito M, Handa Y, Tsubokawa N. et al. Comparison of prognosis between lobectomy and sublobar resection for clinical stage I non-small cell lung cancer with interstitial lung disease. J Thorac Oncol 2017;12:S302–S02. [Google Scholar]
- 40. Amundson WH, Racila E, Allen T, Dincer HE, Tomic R, Bhargava M. et al. Acute exacerbation of interstitial lung disease after procedures. Respir Med 2019;150:30–7. [DOI] [PubMed] [Google Scholar]
- 41. Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis. Clin Chest Med 2012;33:59–68. [DOI] [PubMed] [Google Scholar]
- 42. Lumb AB, Walton LJ.. Perioperative oxygen toxicity. Anesthesiol Clin 2012;30:591–605. [DOI] [PubMed] [Google Scholar]
- 43. Ito H, Nakayama H, Yokose T, Nagashima T, Morohoshi T, Tajiri M. et al. A prophylaxis study of acute exacerbation of interstitial pneumonia after lung cancer surgery. Jpn J Clin Oncol 2020;50:198–205. [DOI] [PubMed] [Google Scholar]
- 44. Iwata T, Yoshino I, Yoshida S, Ikeda N, Tsuboi M, Asato Y. et al. ; West Japan Oncology Group. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ. et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265–75. [DOI] [PubMed] [Google Scholar]
- 46. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J. et al. ; Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its supporting Information files.