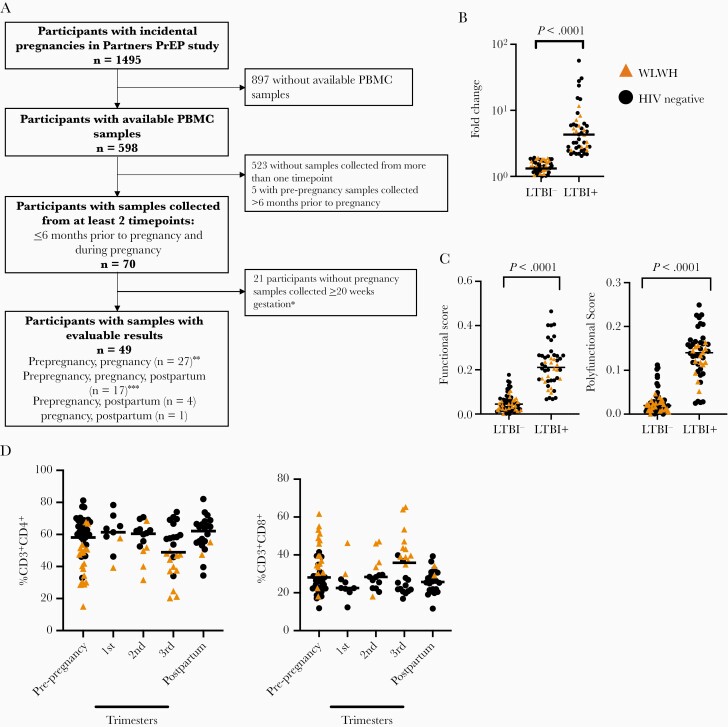
Figure 1.
Baseline immune characteristics are stable across pregnancy. A, Flowchart of stored sample selection of participants with incidental pregnancies in the Partners PrEP study. B, Fold change of the proportion of CD4+ T cells expressing IFN-γ after incubation with ESAT-6 and CFP-10 pooled peptides, compared to vehicle control. C, COMPASS functional score and polyfunctional score stratified by latent tuberculosis infection status. D, Proportion of CD4+ T cells and CD8+ T cells, stratified by pregnancy trimester and HIV status. Prepregnancy sample, n = 49; 1st trimester, n = 9; 2nd trimester, n = 14; 3rd trimester, n = 24; postpartum, n = 22. Circles indicate HIV-negative study participants, while triangles indicate WLWH. Bars indicate median values. ∗Among participants with pre-pregnancy and pregnancy samples only. ∗∗Includes one participant with a sample collected during pregnancy < 20 weeks of gestation without an evaluable postpartum sample. ∗∗∗Includes two participants with two samples collected during pregnancy. Abbreviations: HIV, human immunodeficiency virus; IFN-γ, interferon-γ; LTBI, latent tuberculosis infection; PBMC, peripheral blood mononuclear cell; PrEP, preexposure prophylaxis; WLWH, women living with HIV.