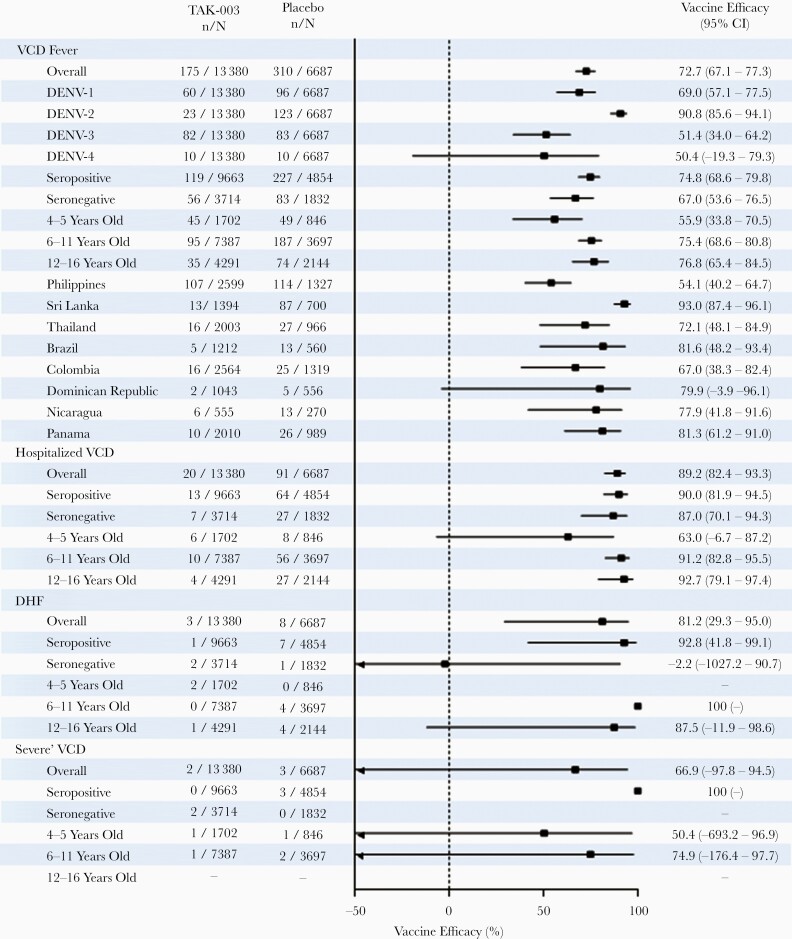
Figure 1.
Vaccine efficacy (95% CI) in prevention of VCD fever, hospitalization due to VCD, severe VCD, and DHF between first dose administration and 12 months after end of part 1 (month 27, year 2 after second dose). Forest plot shows efficacy according to serotype, baseline serostatus, age, and region (safety set data; lower bound 95% CI values < minus 50 not shown on x-axis). For serotype-specific efficacy calculations, only the first instance of VCD due to the individual serotype in question was included, regardless of previous instances of VCD due to other serotypes. Participants were classified as seronegative when testing seronegative for all dengue serotypes at baseline. Participants were classified as seropositive when demonstrating a reciprocal neutralizing antibody titer ≥ 10 against at least 1 dengue serotype at baseline. Cases of severe VCD were determined according to Dengue Case Adjudication Committee criteria. Cases of DHF were determined according to World Health Organization 1997 criteria [23]. Abbreviations: CI, confidence interval; DENV, dengue virus; DHF, dengue hemorrhagic fever; TAK-003, Takeda dengue vaccine candidate; VCD, virologically confirmed dengue.