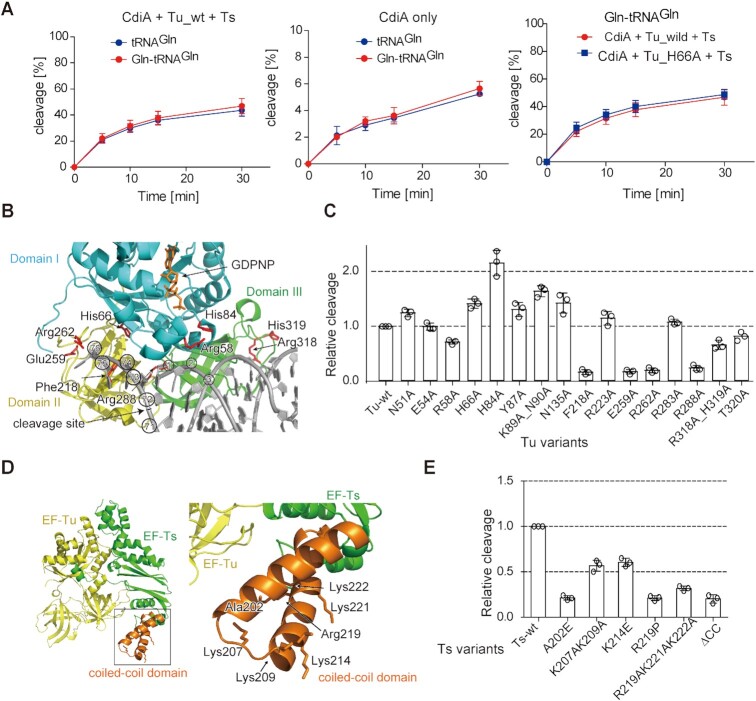
Figure 4.
Involvement of RNA-binding residues in Tu or the coiled-coil domain of Ts. (A) Time courses of tRNAGln and Gln-tRNAGln cleavage by CdiA–CTEC869 in the presence (left) or absence (middle) of Tu, Ts, and GTP at 37°C. For cleavage of Gln-tRNAGln, 1.0 μM of tRNAGln was aminoacylated by GlnRS (1.0 μM) for 60 min before cleavage by CdiA–CTEC869 (0.1 μM) in the absence or presence of translation factors (0.1 μM each) and GTP (1 mM) at pH 7.4. Under this condition, tRNAGln was aminoacylated to the level of 925 pmol/A260. Time courses of Gln-tRNAGln cleavage by CdiA–CTEC869 in the presence of wild-type or H66A mutant Tu (plus Ts, and GTP) (right). (B) Interaction of aa-tRNA with Tu (PDB ID: 1OB2). Domains I, II, and III of Tu are colored cyan, yellow, and green, respectively. Mutated residues are colored red. tRNA is shown as a gray stick model. The nucleotide numbers of tRNA are circled, and the site cleaved by CdiA–CTEC869 is depicted by an arrow. (C) Relative tRNAGln cleavage by CdiA–CTEC869 at 10 min in the presence of a Tu variant, Ts, and GTP. The tRNAGln transcript (1.0 μM) was incubated at 37°C for 10 min with 0.2 μM CdiA–CTEC869 in the presence of a Tu variant (0.2 μM), Ts (0.2 μM), and GTP (1 mM). (D) Crystal structure of the Tu:Ts complex (50). Tu and Ts are colored yellow and green, respectively. The coiled-coil domain in Ts is colored orange. (E) Relative tRNAGln cleavage by CdiA–CTEC869 at 10 min in the presence of Tu, a Ts variant, and GTP. The tRNAGln transcript (1.0 μM) was incubated at 37°C for 10 min with 0.2 μM CdiA–CTEC869 in the presence of Tu (0.2 μM), a Ts variant (0.2 μM), and GTP (1 mM). The tRNAGln cleavage level by CdiA–CTEC869 in the presence of Tu, Ts, and GTP was defined as 1.0 in (C) and (E). The bars in the graphs are SDs of more than three independent experiments, and the data are presented as mean values ± SD.