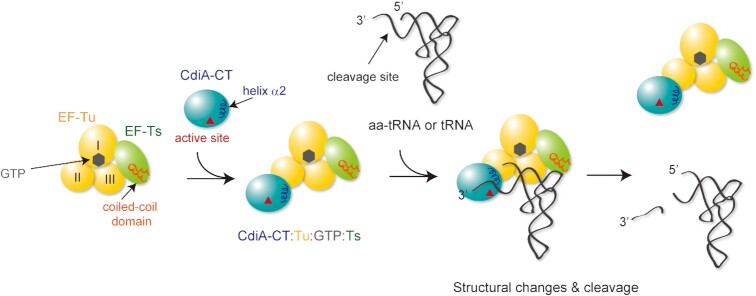
Figure 8.
Model of tRNA cleavage by CdiA–CTEC869 in the presence of translation factors. The Tu:GTP:Ts complex acts as a scaffold for tRNA cleavage by CdiA–CTEC869. First, CdiA–CTEC869 delivered into the cell is recruited to the Tu:GTP:Ts complex to form the CdiA–CT:Tu:GTP:Ts complex. Substrate aa-tRNA (or tRNA) is recognized by CdiA–CT:Tu:GTP:Ts and forms CdiA–CT:Tu:GTP:Ts:aa-tRNA(tRNA). Ts in the CdiA–CT:Tu:GTP:Ts complex increases the affinity of tRNA for the complex and induces a structural change in tRNA and/or CdiA–CT to promote productive catalysis by CdiA–CT. CdiA–CT might interact with Tu:GTP and form CdiA–CT:Tu:GTP, and aa-tRNA (tRNA) could be recognized by the complex. However, aa-tRNA (tRNA) cannot be cleaved by CdiA–CT due to the low affinity of aa-tRNA (tRNA) to the CdiA–CT:Tu:GTP complex. Association of Ts to CdiA–CT:Tu:GTP or association of CdiA–CT to Tu:GTP:Ts is required for aa-tRNA (tRNA) binding and cleavage by CdiA–CT. It is not clear whether CdiA–CT first associates with Tu:GTP and then Ts is recruited to form CdiA–CT:Tu:GTP:Ts or CdiA–CT associates with Tu:GTP:Ts to form CdiA–CT:Tu:GTP:Ts.