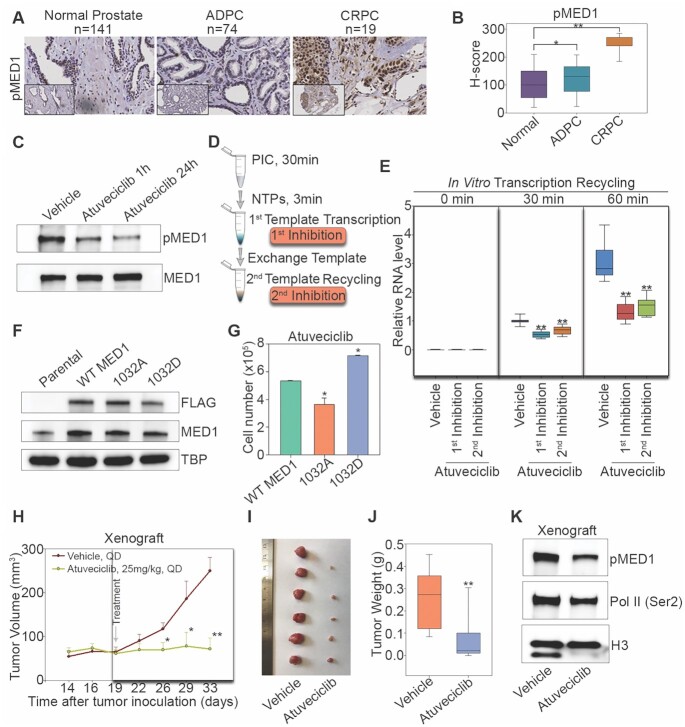
Figure 7.
Pharmacological inhibition of pMED1 suppresses transcription recycling and lethal prostate cancer growth. (A) Representative pMED1 immunoreactivity in normal prostate, ADPC and CRPC tissues. (B) Slides were scanned using an Aperio Digital Pathology Slide Scanner (Leica Biosystems) at 40× magnification and staining was quantified using the Aperio Image Scope (v11). A box plot compares H-scores of pMED1 nuclear staining in 234 tissues. The significance was determined by one-way ANOVA, *P < 0.01, **P < 0.001. (C) LNCaP-abl cells were treated with vehicle or with atuveciclib (1 μM) for 1 or 24 h. Western blot analyses were performed using nuclear extracts with antibodies against pMED1 and MED1. (D) Schematic of the drug-inhibited transcription recycling assay. Atuveciclib (1 μM) was added either during first template transcription after 3 min of NTP addition or during second template recycling after exchange of template. (E) RNA products from the second template reaction were analyzed by reverse transcription followed by qPCR quantification. Regions = 7 (n = 2), P-values were calculated using two-tailed Student’s t-test, *P < 0.01, **P < 0.001. (F) Western blots of lysates from LNCaP-abl cells transiently expressing WT or mutated FLAG-MED1 proteins. (G) Cell proliferation with atuveciclib treatment was measured by direct cell counting assays. Results are mean ± SD of duplicate experiments. The significance was determined by one-way ANOVA, *P < 0.05. (H) Tumor growth in mice treated with atuveciclib (n = 11) or vehicle (n = 11) once daily. Treatments were started 19 days after inoculation and continued for 14 days. Data are represented as mean ± SEM. Significance was calculated using two-tailed Student’s t-test, *P < 0.05, **P < 0.001. (I) Representative picture of vehicle- and atuveciclib-treated LNCaP-abl tumors at time of collection. (J) Tumor weight for vehicle (n = 11) and atuveciclib (n = 11) groups. The significance was determined by two-tailed Student’s t-test, **P < 0.001. (K) Western blots were performed using nuclear extracts from pooled xenograft tissues from vehicle- (n = 2) or atuveciclib-treated mice (n = 6) with the indicated antibodies.