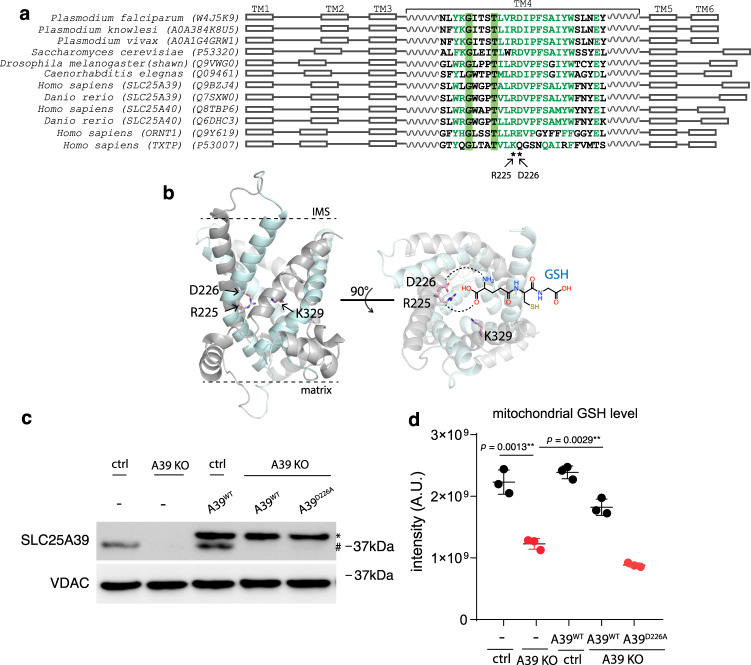
Fig. 5. Structural modeling and functional mutagenesis of SLC25A39.
a Multiple sequence alignment of the eukaryotic A39 ortholog sequences spanning diverse taxa alongside with the human amino acid ornithine transporter SLC25A15 (ORNT1) and the human citrate transporter SLC25A1 (TXTP). The positions for the human SLC25A39 Arg225 and Asp226 residues predicted to bind amino acids are labeled by asterisks. b The modeled human A39 structure based on the ANT structure c-state conformation (PDB: 1OKC), in both side view (left) and cytoplasmic/mitochondrial intermembrane space (IMS) view (right). Odd transmembrane domain (TM1, 3, 5) are shown in gray and even (TM2, 4, 6) are shown in cyan. Two predicted GSH binding residues (R225, D226) and the predicted residue critical for solute-binding induced conformational change (K329) are shown. The predicted interaction between R225 and D226 with the carboxylate and amino group of the glutamate residue of GSH is shown by dashed line. c Western blotting showing the endogenous A39 (#) and ectopically expressed A39 protein (*). VDAC was used as the loading control. d LC-MS measurement of mitochondrial GSH level showing that the predicted substrate binding mutant A39D226A cannot restore mitochondrial GSH level in the A39 KO cells (n = 3). Statistical significance was calculated using two-tailed t test. Significance level were indicated as *** p < 0.001, ** p < 0.01, * p < 0.05 and n.s. p > 0.05. Data are expressed as mean ± SD. Source data are provided as a Source Data file.