Abstract
Recently, pharmaceutical scientists' interest has increased to find novel pharmaceutical natural substances with potent antioxidant capacity and very low side effects to be used safely in preventive medicine. One of the most common types of diseases with a large spread globally is cardiovascular diseases, which cause a high rate of deaths annually. The present study evaluated the use of Artemisia herba alba leaves’ extract (AHALE) and AHALE zinc oxide nanoparticles (AHALE–ZnONPs) against isoproterenol (ISO) inducing myocardial infarction (MI) in male rats. Several groups of Wistar male rats fed a high-fat diet (HFD) were pretreated with several doses of AHALE or AHALE–ZnONPs for one month followed by exposure to ISO for two days. After treatment, samples of the rats’ heart tissues and blood were collected for several molecular biological and biochemical analyses. Heart enzymes, antioxidant enzymes, lipid peroxidation compounds, lipid markers, activities, ROS generation, apoptosis, DNA damage and expression of lipid metabolism genes were analyzed in rats pretreated with AHALE or AHALE–ZnONPs followed by exposure to ISO. The results showed an increase in the levels of AST, ALT, LDH, CK, CK-MB, and cTnT (heart markers), elevation in TG, TC, and LDL levels (lipid profile markers), levels of TBARS and LOOH (lipid peroxidation products), ROS generation, DNA damage, apoptosis, and upregulation of PPAR-α, ADD1, FASN, and ACC genes in animals exposed to ISO in comparison with the control animals. Moreover, a decrease in antioxidant enzyme activities, including GPx, GRx, and GST, was observed in animals exposed to ISO in comparison with control rats. In male rats pretreated with AHALE or AHALE–ZnONPs followed by exposure to ISO, the oxidative stress induced by ISO was prevented. The results suggest that Artemisia extract could be considered for use as one of the natural compounds for prevention of atherosclerosis and heart diseases due to its high antioxidant and hypolipidemic activities. The reduced oxidative stress of Artemisia extract may be a result of the existence of flavonoids and phenolic substances.
Keywords: Myocardial infarction, Isoproterenol, Artemisia herba alba, Lipid profile, Cardiac markers, ROS generation, Apoptosis
1. Introduction
Heart disease is very common and a major cause of death worldwide. Myocardial infarction (MI) is the most serious heart problem. It is common in patients with ischemic heart disease. Its occurrence is attributed to the sudden and continuous interruption of the blood supply to the heart muscle, which leads to necrosis of the heart muscle (Anversa and Sonnenblick, 1990, Sandoval and Jaffe, 2019). An MI is often responsible for many other health problems as a result of pathophysiological and biochemical alterations including high blood sugar, peroxidation of lipids, high blood lipids, etc. (Anversa and Sonnenblick, 1990). Among several drugs dangerous for the heart, isoproterenol (ISO), a synthetic catecholamine causes an MI when used in high doses. The ISO causes cardiac damage as a result of its participation in the generation of free radicals that are highly toxic to heart cells, through the self-oxidation of catecholamines. It was also found that the excessive production of ROS may lead to a loss of the integrity and role of the myocardial membranes (Biemond et al., 1986, Iqbal et al., 2019, Iqbal et al., 2019).
Measurements of cardiac marker enzymes are an important method for detecting cardiac injury. The most important markers are cardiac creatine kinase (CK), CK-MB, lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine transaminase (ALT) found in patients' blood materials (Mair, 1997). One of the important and sensitive indicators for detecting the status of MI is Toponin-T (cTnT). cTnT is a protein that is released from the heart to cause contractions when myocardial necrosis occurs, so it is not normally found in blood serum (Katus et al., 1991).
The lipid oxidation process, (the oxidative degradation of polyunsaturated fatty acids, PUFAs) normally occurs by changing the structure of cell membranes and inhibiting antioxidant enzymes. It has been found that at the initial stage of cell and tissue oxidation, there is an increase in lipid oxidation levels in the form of TBARS (thiobarbituric acid reactive substances) and LOOH (lipid hydroperoxides). On the other hand, there is a functional correlation between antioxidants and free radical generation, where antioxidants play an important role in effectively removing ROS from cells by antioxidant enzymes removing free radicals (Liu et al., 2021). The important antioxidants in this function are GRx (glutathione reductase), GPx (glutathione peroxidase), and GST (glutathione-S-transferase) (Harrison et al., 2003). They are considered the first line of defense for the cells against oxidative damage. However, it was found that the higher the lipid peroxidation process, the lower the activity level of these enzymes.
Medical and pharmacologists have discovered in recent years that there is a link between eating foods that contain antioxidants, such as fresh vegetables, fruits and medicinal herbs, and the prevention of cardiovascular diseases (Argolo et al., 2004, Román et al., 2019). The protective roles of these natural materials could be attributed to their content of anthocyanins, phenolic compounds, and flavonoids (Sanchez-Moreno et al., 1998, Zhang and Wang, 2002).
One of the most important medicinal plants that is abundant in Arabian countries is Artemisia herba alba, which is used in traditional medicine against many diseases (Radulović et al., 2013). This plant contains a high concentration of antioxidants, cineole, artemisia ketone, and camphene (Skowyra et al., 2014). As a result of containing a high percentage of phenolic compounds and flavonoids, it has a high ability to prevent the generation of ROS (Agate et al., 2009). In order to achieve the most benefit from the extract of the plant Artemisia herba alba, it has been converted into nanoparticles coated on Zno nanoparticles, which are the nanoparticles used in the manufacture of cosmetic creams that protect the skin from carcinogens (Shalyapina et al., 2012). Recently, use of herbal remedies may have an important role in improving cardiomyopathy. Atale et al. (2017) investigated the protective effects of AgNPs + extract of S. cumini seeds on cardiac cells of rats. The results showed the ability of AgNPs + extract of these seeds to reduce lipid peroxidation and cellular stress, more effectively than using the extract alone. The ability of AgNPs + extract of S. cumini seeds is attributed to the presence of polyphenolic compounds with strong antioxidant activity (Neha et al., 2013). Thus, the main aim of this study was to investigate the protecting role of AHALE and AHALE–ZnONPs against ISO inducing myocardial infarction in rats.
2. Materials and methods
2.1. Sampling of plant materials
A. herba alba leaves were collected from the Tabuk region, Saudi Arabia. After the process of collecting and transferring the samples to the laboratory, the plant materials were dried using solar energy.
2.2. Plant extract preparation
Dry leaves of the plant (100 g) were used to obtain the plant extract from each group collected separately by using ethyl alcohol (70%) in a volume of 500 ml. The samples were placed with the alcohol solution in a shaker at room temperature for three days. Then, a centrifugation was carried out to obtain the precipitate containing the plant extract. The upper phase containing the suspended residues in the alcohol solution was used several times after centrifugation to separate the extract again. The collected materials after centrifugation were then mixed and concentrated at 40 °C under reduced pressure. Ethanol alcohol was evaporated and the dried extracts were collected in sterile tubes and kept at 4 °C until use (Alshehri et al., 2019).
2.3. ZnO nanoparticles biosynthesis
To prepare ZnO-NPs in a concentration of 1 mM with AHALE, an aqueous extract solution of fresh AHALE was added to the ZnO-NPs at a ratio of 9:1 (v:v). The mixture of ZnONPs and AHALE solution was placed in a shaker at 28 ± 3 °C for several hours with constant rotation (Prasannaraj and Venkatachalam, 2017).
2.4. AHALE–ZnONPs characterization
Using UV–visible spectrophotometry, the prepared AHALE–ZnONPs were characterized. To demonstrate the biosynthesis of AHALE–ZnONPs, UVD 3200-UV–visible spectrophotometry (Labomed, Los Angeles, CA 90034 U.S.A) was utilized. An X-ray diffraction meter (Equinox 3000) was used to evaluate the X-ray diffraction of AHALE–ZnONPs. Moreover, to analyze the size and shape of AHALE–ZnONPs, Hitachi electron microscopy (S-4160) was used. Finally, the Nno-z 590 Malvern–Zetasizer was used to measure the particle sizes of the prepared AHALE–ZnONPs.
2.5. Induction of myocardial infarction in experimental animals
A solution of isoproterenol (ISO) was prepared in which ISO was dissolved in saline solution. The animals were injected subcutaneously with a concentration of 100 mg/kg b.wt. once daily for two days to induce an experimental MI.
2.6. Experimental animals
Animals (adult Wistar male rats, n = 80, 145–160 g), were fed a regular diet and placed into eight groups (10 rats each) in separate plastic cages. Following the animal care guidelines in accordance with the Committee of Ethics at the College of Science of Tabuk University, Saudi Arabia, an experimental protocol was applied through which the rats did not suffer at any time during the experiment. All protocols and procedures concerning animal handling and care of animals (NIH guidelines) followed to the ARRIVE guidelines (Kilkenny et al., 2010) were taken into consideration.
2.7. Treatment protocol
Eight groups of animals (10 rats each) were used in this study. The experimental groups were designed as follows: Group 1: Normal untreated rats were fed ad libitum. Group 2: Rats were fed a high-fat diet (HFD, containing 3% cholesterol for four weeks) (El-Tantawy, 2015) followed by treatment with isoproterenol (ISO, 100 mg/kg b.wt.) to induce MI (Priscilla and Prince, 2009). Groups 3–5: Rats fed an HFD were pretreated with AHALE (50, 150, and 300 mg/kg b.wt., respectively) orally for four weeks and then injected with ISO for two days (AL-Ibrahemi et al., 2020). Groups 6–8: Animals fed an HFD were pretreated with AHALE–ZnONPs (with similar doses to those in groups 3–5, respectively) orally for four weeks and then injected with ISO for two days (AL-Ibrahemi et al., 2020). Several doses of nanoparticles plus plant extracts were used to assess the best dose for therapy of cardiomyopathy with low side effects.
One day after the final ISO exposure, animals were anesthetized and euthanized. Blood samples were taken and immediately used for separation of serum samples. Heart samples were collected instantly and placed in fresh prepared saline solution. The heart tissues were divided into (a) one part for estimation several biochemical parameters including antioxidant enzymes, lipid peroxidation compounds, lipid markers, ROS generation, and apoptosis; and (b) the second part stored at −80 °C for determination of DNA damage and expression of lipid metabolism genes analyses.
2.8. Biochemical analyses
2.8.1. Analysis of heart marker enzymes
Using commercial kits for heart markers, the activities of CK and CK-MB were measured in rat serum samples. According to Reitman and Frankel (1957), serum ALT, AST, and LDH activities were colorimetrically measured using the Quimica Clinica Aplicada kit (Spain).
2.8.2. Lipid profile assessment
Serum samples were used to determine the total cholesterol (TC) amounts, triglyceride (TG) levels, and high density lipoprotein (HDL) values in all treated groups. Levels of HDL were estimated using Biodiagnostic kits, KSA. HDL levels were colorimetrically measured at a wavelength of 500 nm.
2.8.3. cTnT estimation
A chemiluminescence immunoassay standard kit (Roche Diagnostics, Switzerland) was used to determine the levels of cTnT in the serum samples of treated groups.
2.8.4. Antioxidant and lipid peroxidation product estimation
Lipid peroxidation substances (TBARS) in the heart tissues were determined according to Fraga et al. (1988). Moreover, levels of LOOH were estimated according to Jiang et al. (1992). GST, GRx, and GPx activities were analyzed according to the methods of Habig and Jakoby, 1981, Horn and Burns, 1978, and Rotruck et al. (1973), respectively.
2.8.5. Comet assay
DNA damage estimation in the treated rats’ heart tissues was carried out using comet analysis according to Blasiak et al. (2004). The class of the damaged DNA in the tail was evaluated in three categories from class 1 to class 3.
2.8.6. Determination of apoptotic cells
Measurement of apoptosis was conducted according to Villalba et al. (1992). The heart tissues were homogenized to make the single-cell suspensions necessary to measure the apoptosis for individual cells. To determine the apoptosis levels in the suspensions of treated cells through flow cytometry, an Annexin V/PI apoptosis detection kit was used.
2.8.7. ROS generation assessment
ROS formation determination in the heart samples of treated animals was evaluated with a flow cytometer using a fluorescent probe according to, Khalil et al., 2018. Assessment of the signals of ROS formation was carried out at 525 nm emission and 488 nm excitation.
2.8.8. Expression profile of lipid related genes
TRIzol® reagent was used to extract the total RNA of heart tissues according to the isolation manual of the reagent. The isolated RNA was kept in DEPC water and was stored in aliquots at −80 °C (Salem et al., 2018). A kit for cDNA Synthesis was utilized to synthesize the cDNA copy using the template RNA via reverse transcription reaction (Khalil, et al.,2019. The former copies of synthesized cDNA were used for a real-time reaction using the SYBR® Premix Ex TaqTM kit. The obtained CT values from qRT-PCR reactions using specific primers for the PPAR-α, ADD1, FASN, and ACC genes (Table 1) were normalized on the CT values of β-actin gene using the 2−ΔΔCT method.
Table 1.
Sequences of primers used in the gene expression analysis.
| Gene | Sequence of Primers (5′–3′) | Accession No.(GenBank) |
|---|---|---|
| PPAR-α | F- AGC CTC TTT GCC CAG ATC TT R- GCA ATC CGT CTT CAT CCA CC |
NM_031347.1 |
| ADD1 | F- CCC ACC TCA AAC CTG GAT CT R- TCA GTG CCA GGT TAG AAG CA |
L16995.1 |
| FASN | F- TGG TGA TAG CCG GTA TGT CC R- TCA GCT TTC CAG ACC GCT TA |
NM_017332.2 |
| ACC | F- ATG TGC AAT GAG ACC CCT GA R- AGG AAT CCA AGA TGA GCC CC |
AI716652.1 |
| β-actin | F- AGG GTG TGA TGG TGG GTA TG R- TCA TCT TTT CACGGT TGG CC |
C06968.1 |
F: forward primer; R: reverse primer; Lipid metabolism-related transcriptional regulators: PPAR-α, ADD1, FASN, and ACC.
2.9. Statistical analysis
The biochemical and genetic parameters data obtained in this study were statistically analyzed using GLM (General Liner Models) from the software of SAS (Statistical Analysis System). Moreover, the Scheffé test was used to assess the considerable differences between treatments. The data obtained from the analyzed parameters are illustrated as the average ± SEM. Furthermore, a probability of P < 0.05 was used to estimate the significance statements between treatments.
3. Results
3.1. Characterization of ZnO-NPs
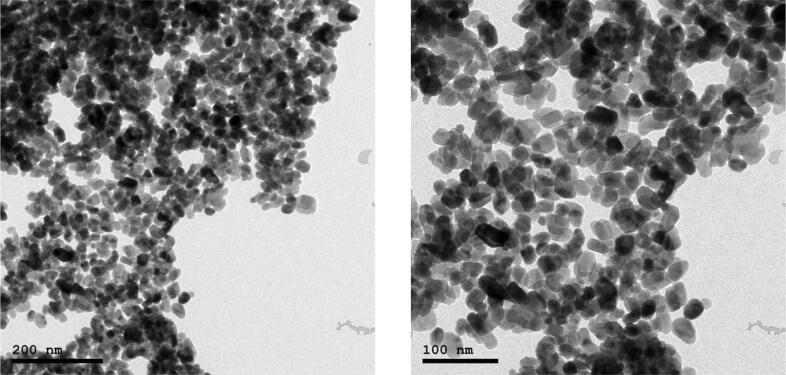
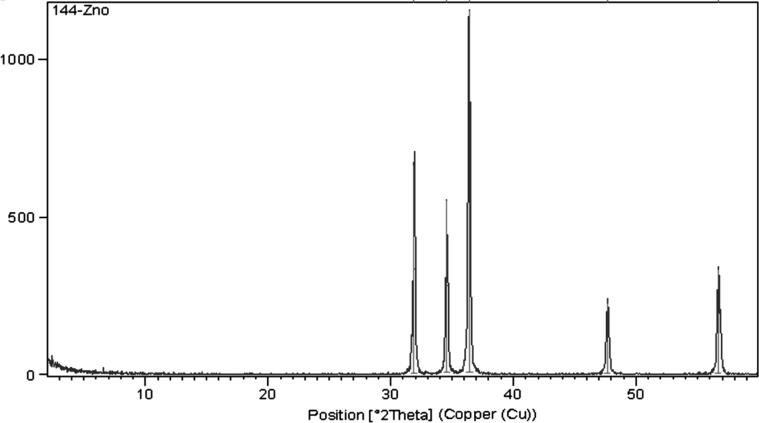
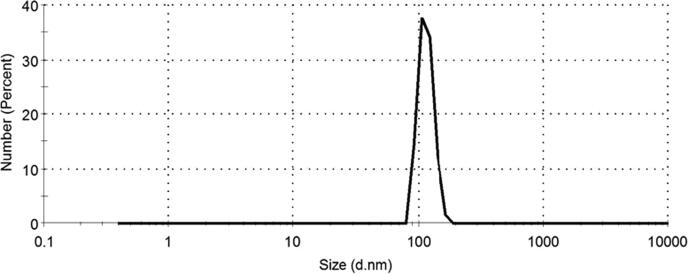
The morphological and structural properties of the as-prepared ZnO-NPs were confirmed by using TEM and XRD as shown in Fig. 1, Fig. 2, respectively. TEM micrograph shows that ZnO-NPs are quasi-spherical shape with a relatively narrow size distribution. The average particle size of the as-prepared ZnO-NPs is distributed in the range from 25 ± 5 nm, as shown in Fig. 1. In addition, XRD patterns as depicted in Fig. 2 showed that ZnO-NPs exhibited perfect Zancite (i.e., Hexagonal) crystallographic structure. Furthermore, the hydrodynamic diameter (HD) in a vehicle solution, for as-prepared ZnO-NPs was about 114.8 ± 16.12 nm with high polydispersity index (PDI) 0.89, as shown in Fig. 3.
Fig. 1.
TEM image of ZnO nanoparticles at different scale (20 ± 5 nm).
Fig. 2.
XRD Patterns of ZnO NPs.
Fig. 3.
DLS data of ZnO nanoparticles with hydrodynamic diameter (HD) = 114.8 ± 16.12 nm, and polydispersity index (PDI) is 0.89.
3.2. Estimation of cardiac markers
Serum ALT, AST, LDH, CK, and CK-MB activities in rats pretreated with AHALE and AHALE–ZnONPs followed by ISO induction are presented in Table 2. ISO treatment exhibited high activities of serum cardiac markers of the male rats compared to the control rats. However, pretreatment of male rats with AHALE at medium and high doses decreased considerably the activities of heart markers in MI rats induced by ISO exposure in comparison with rats exposed to ISO only. Moreover, pretreatment of male rats with all three doses of AHALE–ZnONPs significantly decreased the activities of heart markers in MI rats induced by ISO in comparison with rats exposed to isoproterenol only.
Table 2.
Effect of AHALE and AHALE-ZnONPs on heart markers levels in MI induced rats.
| Treatment | ALT (U/L) | AST (U/L) | LDH (U/L) | CK (U/L) | CK-MB (U/L) |
|---|---|---|---|---|---|
| Control | 25.18 ± 2.2 d | 36.11 ± 4.7c | 81.52 ± 9.1 e | 158.14 ± 13.2 e | 79.52 ± 8.4 d |
| Isoproterenol | 48.12 ± 3.9 a | 58.19 ± 6.3 a | 159.26 ± 15.4 a | 291.18 ± 17.6 a | 186.63 ± 12.5 a |
| AHALE50 + ISO | 44.16 ± 5.8 a,b | 54.32 ± 5.2 a,b | 153.40 ± 12.8 a | 275.16 ± 18.2 a | 169.72 ± 14.7 a |
| AHALE150 + ISO | 38.92 ± 7.6b | 49.94 ± 6.3b | 136.27 ± 13.2b | 237.11 ± 14.3b | 148.55 ± 9.2b |
| AHALE300 + ISO | 31.50 ± 3.7c | 42.35 ± 4.8b,c | 120.34 ± 10.9c | 219.22 ± 15.8c | 121.40 ± 8.8c |
| AHALE-ZnONPs 50 + ISO | 39.22 ± 5.2b | 48.53 ± 5.9b | 142.70 ± 11.7b | 251.13 ± 16.4 a,b | 158.73 ± 10.6 a,b |
| AHALE-ZnONPs 150 + ISO | 33.41 ± 4.5c | 43.62 ± 4.1b,c | 121.51 ± 8.5c | 214.91 ± 13.6c | 122.46 ± 9.2c |
| AHALE-ZnONPs 300 + ISO | 27.22 ± 2.8 d | 39.25 ± 4.6c | 102.77 ± 6.1 d | 186.72 ± 11.4 d | 93.22 ± 6.5 d |
MI: Myocardial infarction; AHALE: A. herba alba leaves extract; ZnONPs: Zinc Oxide nanoparticles; 50, 150, and 300: doses of AHALE or AHALE-ZnONPs; Data are presented as mean ± SEM. a,b,c,d,e Mean values within treatment with different superscript letters were significantly different (p < 0.05).
3.3. Lipid profile
Table 3 shows the alteration in lipid profile (TG, TC, HDL, and LDL) in rats pretreated with AHALE and AHALE–ZnONPs followed by isoproterenol exposure. Rats exposed to ISO exhibited considerable elevation in the TG, TC, and LDL levels as well as a significant decrease in HDL levels compared to the control animals. However, pretreatment of rats with AHALE at a high dose followed by exposure to isoproterenol decreased significantly the TG, TC, and LDL levels and elevated the levels of HDL compared to the rats exposed to isoproterenol only. Furthermore, pretreatment of rats with AHALE–ZnONPs at the medium and high doses followed by exposure to isoproterenol decreased significantly the TG, TC, and LDL levels as well as elevating the levels of HDL compared to those in animals exposed to isoproterenol only.
Table 3.
Lipid profile in rats pretreated with AHALE and AHALE-ZnONPs followed by ISO exposure.
| Treatment | TG (mg/dl) | TC (mg/dl) | HDL (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|
| Control | 63.2 ± 3.1 d | 78.5 ± 3.4c | 31.2 ± 3.4 a | 43.1 ± 2.1 e |
| Isoproterenol | 95.6 ± 5.7 a | 129.6 ± 7.1 a | 22.1 ± 1.8c | 91.4 ± 3.5 a |
| AHALE50 + ISO | 89.2 ± 6.4 a | 118.4 ± 6.6 a | 22.9 ± 2.2c | 87.5 ± 2.7 a |
| AHALE150 + ISO | 81.4 ± 6.8b | 103.1 ± 5.3b | 23.7 ± 1.3b,c | 78.7 ± 2.2b |
| AHALE300 + ISO | 76.8 ± 4.5b,c | 97.5 ± 6.1b,c | 25.4 ± 2.4b | 64.2 ± 1.8c |
| AHALE-ZnONPs 50 + ISO | 83.5 ± 5.1 a,b | 106.2 ± 5.8b | 23.5 ± 3.6b,c | 79.6 ± 3.4b |
| AHALE-ZnONPs 150 + ISO | 74.9 ± 4.9c | 95.1 ± 4.9b,c | 27.2 ± 1.5 a,b | 66.1 ± 2.6c |
| AHALE-ZnONPs 300 + ISO | 66.3 ± 2.5 d | 83.6 ± 3.3c | 29.7 ± 3.1 a | 52.4 ± 1.3 d |
MI: Myocardial infarction; AHALE: A. herba alba leaves extract; ZnONPs: Zinc Oxide nanoparticles; 50, 150, and 300: doses of AHALE or AHALE-ZnONPs; Data are presented as mean ± SEM. a,b,c,d,e Mean values within treatment with different superscript letters were significantly different (p < 0.05).
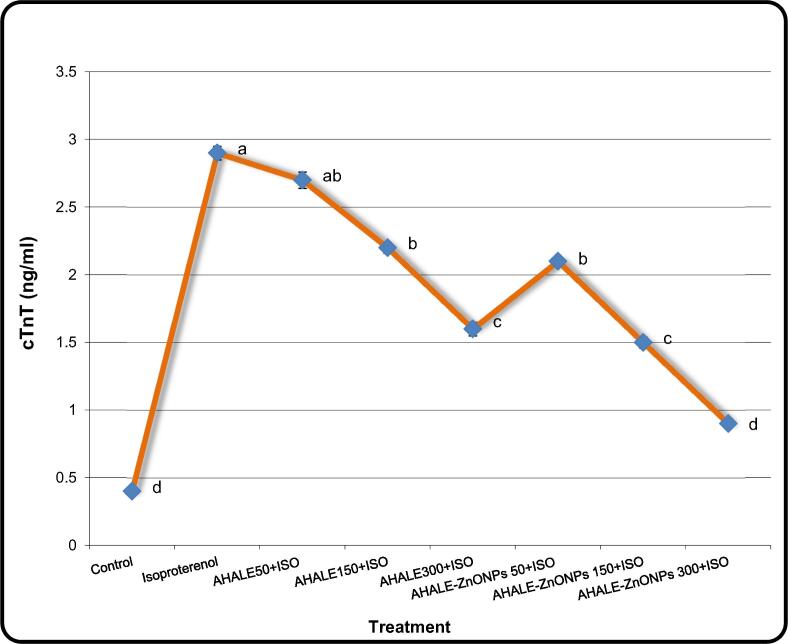
3.4. Cardiac troponin-T (cTnT) levels
Fig. 4 shows the cTnT levels in the serum of rats pretreated with AHALE and AHALE–ZnONPs followed by isoproterenol. Male rats exposed to ISO exhibited high levels (P < 0.05) of cTnT in comparison with those in normal animals. In contrast, a considerable decrease in the cTnT levels was found in ISO-exposed rats pretreated with medium and high doses of AHALE as well as with all three doses of AHALE–ZnONPs compared to those exposed to ISO only.
Fig. 4.
The levels of cTnT in the serum of rats pretreated with AHALE and AHALE-ZnONPs followed by ISO exposure. Results are expressed as the mean ± SD. a,b,c,d Mean with different letters, within treatment, differs significantly (p < 0.05).
3.5. Peroxidated products and activity of antioxidants
Rats exposed to ISO showed high TBARS and LOOH levels in heart tissues in comparison with normal rats (Table 4). In contrast, rats exposed to ISO and pretreated with AHALE (at medium and high doses) and AHALE–ZnONPs (at all three doses) showed considerable (P < 0.05) decline in TBARS and LOOH levels compared to those exposed to ISO only (Table 4).
Table 4.
Lipid peroxidation products and enzymatic antioxidants activities in rats pretreated with AHALE and AHALE-ZnONPs followed by ISO exposure.
| Treatment | TBARS | LOOH | GPx | GRx | GST |
|---|---|---|---|---|---|
| Control | 1.72 ± 0.11 d | 27.15 ± 2.4 d | 6.21 ± 0.7 a | 8.73 ± 0.6 a | 792.53 ± 34.5 a |
| Isoproterenol | 8.41 ± 0.84 a | 69.42 ± 7.2 a | 2.47 ± 0.2c | 3.51 ± 0.1 d | 389.42 ± 27.3 e |
| AHALE50 + ISO | 7.63 ± 0.63 a,b | 63.12 ± 6.1 a,b | 2.68 ± 0.3c | 3.96 ± 0.2c,d | 402.31 ± 38.2 d,e |
| AHALE150 + ISO | 6.29 ± 0.52b | 57.91 ± 5.2b | 3.15 ± 0.4b,c | 4.59 ± 0.3c | 457.82 ± 41.7 d |
| AHALE300 + ISO | 4.88 ± 0.17c | 49.27 ± 4.3c | 4.52 ± 0.3b | 5.79 ± 0.2b,c | 525.60 ± 35.1c |
| AHALE-ZnONPs 50 + ISO | 6.83 ± 0.40b | 56.82 ± 3.3b | 3.19 ± 0.2b,c | 4.52 ± 0.1c | 435.52 ± 46.6 d |
| AHALE-ZnONPs 150 + ISO | 4.21 ± 0.35c | 48.72 ± 4.1c | 4.46 ± 0.5b | 5.38 ± 0.4b,c | 537.15 ± 29.4c |
| AHALE-ZnONPs 300 + ISO | 3.11 ± 0.14c | 35.65 ± 3.9 d | 5.37 ± 0.6 a,b | 6.51 ± 0.5b | 655.91 ± 44.2b |
TBARS (mmol/100 g wet tissue); LOOH (mmol/100 g wet tissue); GPx (µg of GSH oxidized/min/mg protein); GRx (nmol of NADPH oxidized/min/100 mg protein); GST (nmol of CDNB conjugated/min/mg protein); MI: Myocardial infarction AHALE: A. herba alba leaves extract; ZnONPs: Zinc Oxide nanoparticles; 50, 150, and 300: doses of AHALE or AHALE-ZnONPs; Data are presented as mean ± SEM. a,b,c,d,e Mean values within treatment with different superscript letters were significantly different (p < 0.05).
The antioxidant activities of enzymes including GPx, GRx, and GST were considerably decreased in the heart tissues of rats exposed to ISO compared to control rats (Table 4). However, the GPx, GRx, and GST activities in rats exposed to ISO and pretreated with AHALE (at medium and high doses) and AHALE–ZnONPs (at all three doses) were significantly (P < 0.05) elevated in comparison with those treated with ISO only (Table 4).
3.6. DNA damage and apoptosis
Rats exposed to ISO exhibited significant elevation in the rate of DNA damage in heart tissues in comparison with those in normal animals (Table 5). In contrast, rats pretreated with AHALE (at medium and high doses) and AHALE–ZnONPs (at all three doses) and then exposed to ISO showed a significant decrease in the rate of DNA damage compared to those exposed to ISO only (Table 5).
Table 5.
The impact of AHALE and AHALE-ZnONPs on the damaged DNA in MI induced rats.
| Treatment | No. of Cells |
Class¥ of Comet |
DNA Damaged Cells (Mean ± SEM) | ||||
|---|---|---|---|---|---|---|---|
| Analyzed | Total Comets | 0 | 1 | 2 | 3 | ||
| Control | 500 | 34 | 466 | 29 | 5 | 0 | 6.81 ± 0.67 d |
| Isoproterenol | 500 | 116 | 384 | 37 | 38 | 41 | 23.20 ± 0.74 a |
| AHALE50 + ISO | 500 | 108 | 392 | 39 | 35 | 34 | 21.62 ± 1.08 a,b |
| AHALE150 + ISO | 500 | 91 | 409 | 34 | 31 | 26 | 18.24 ± 0.58b |
| AHALE300 + ISO | 500 | 67 | 433 | 25 | 20 | 22 | 13.44 ± 0.60c |
| AHALE-ZnONPs 50 + ISO | 500 | 88 | 412 | 30 | 32 | 26 | 17.63 ± 0.93b |
| AHALE-ZnONPs 150 + ISO | 500 | 69 | 431 | 23 | 25 | 21 | 13.80 ± 0.92c |
| AHALE-ZnONPs 300 + ISO | 500 | 48 | 452 | 19 | 16 | 13 | 9.64 ± 0.51 d |
: Class 0 = no tail; 1 = tail length < diameter of nucleus; 2 = tail length between 1X and 2X the diameter of nucleus; and 3 = tail length > 2X the diameter of nucleus. (*): No. of cells analyzed were 100 per animal. MI: Myocardial infarction AHALE: A. herba alba leaves extract; ZnONPs: Zinc Oxide nanoparticles; 50, 150, and 300: doses of AHALE or AHALE-ZnONPs; Data are presented as mean ± SEM. a,b,c,d Mean values within treatment with different superscript letters were significantly different (p < 0.05).
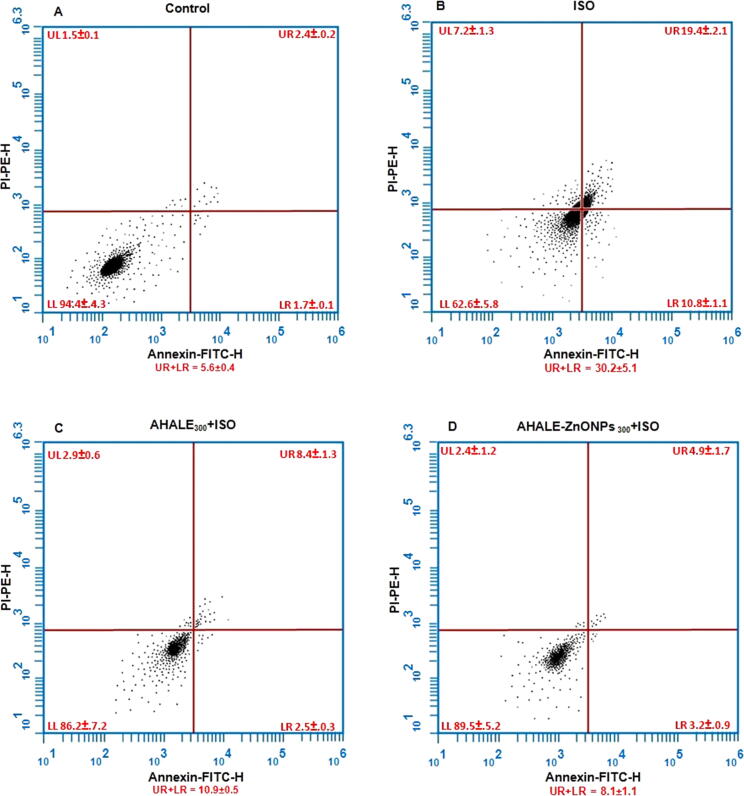
The apoptosis rates in rats exposed to ISO were increased (30.2 ± 5.1) compared with those (5.6 ± 0.4) in control rats (Fig. 5). In contrast, animals pretreated with a high dose of AHALE and AHALE–ZnONPs followed by exposure to ISO showed a significant decrease in the rates of DNA damage (10.9 ± 0.5 and 8.1 ± 1.1, respectively) compared to those exposed to ISO only.
Fig. 5.
Flow cytometry analysis of annexin-V-FITC of (A) control and rats treated with (B) ISO, (C) AHALE300 + ISO, and (D) AHALE-ZnONPs 300 + ISO. The upper left quadrant (UL) represents necrotic cells, the left lower quadrant (LL) represents healthy cells, the upper right quadrant (UR) represents early apoptotic cells, and the lower right quadrant (LR) represents late apoptotic cells. Apoptosis was calculated as summation of UR + LR. Values represent mean percentage ± SEM of at least three samples.
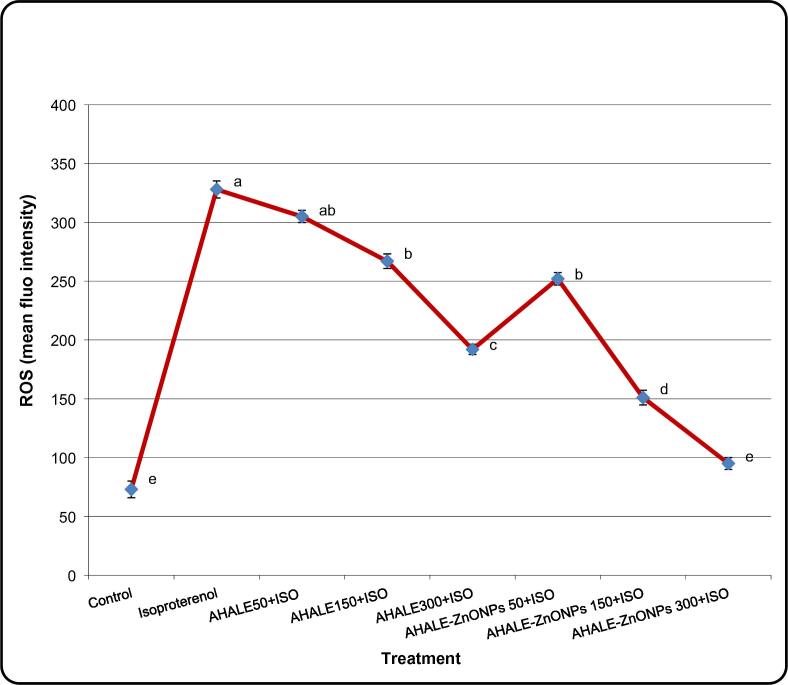
3.7. ROS generation
Formation of ROS levels in the heart tissues of animals treated with ISO was substantially (P < 0.05) increased in comparison with those in normal rats (Fig. 3). Nevertheless, rats pretreated with AHALE (at medium and high doses) and AHALE–ZnONPs (at all three doses) followed by exposure to ISO showed significant decrease in ROS generation compared to those exposed to ISO only (Fig, 6).
3.8. Expression levels of genes encoding pathway of lipids
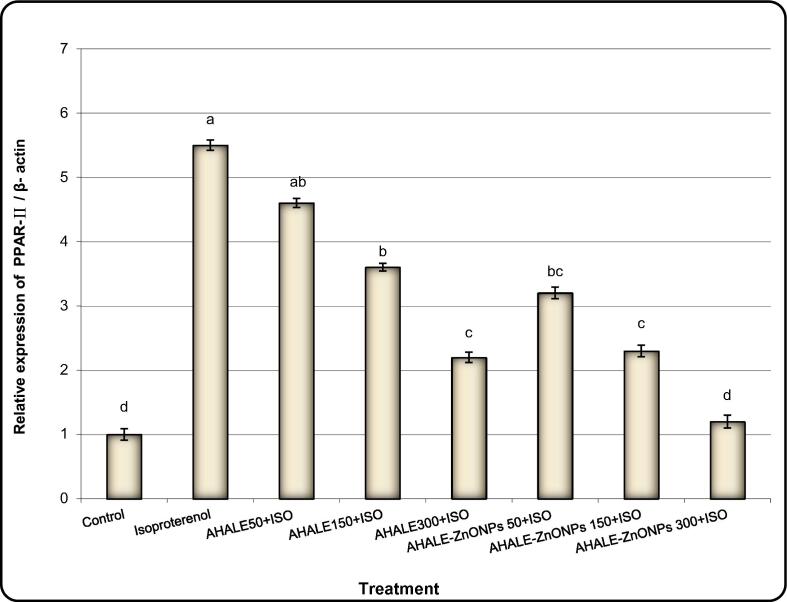
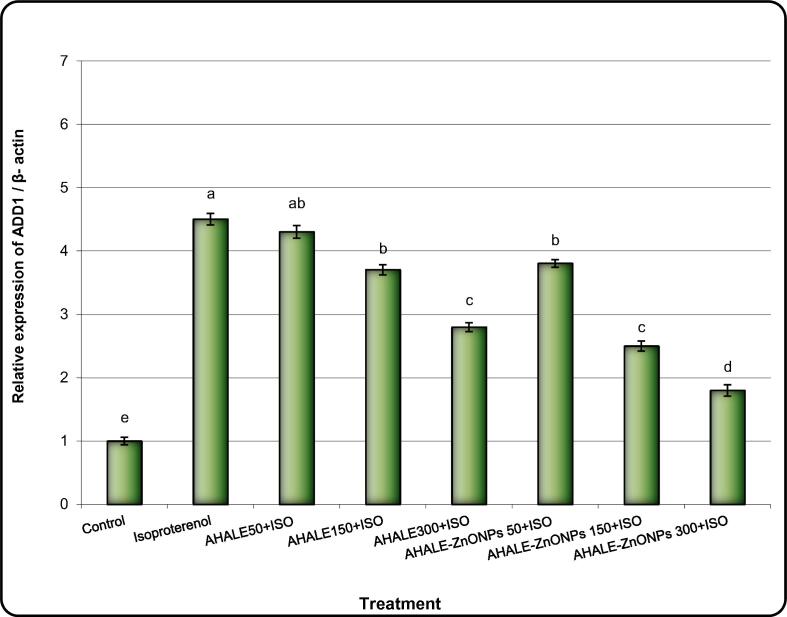
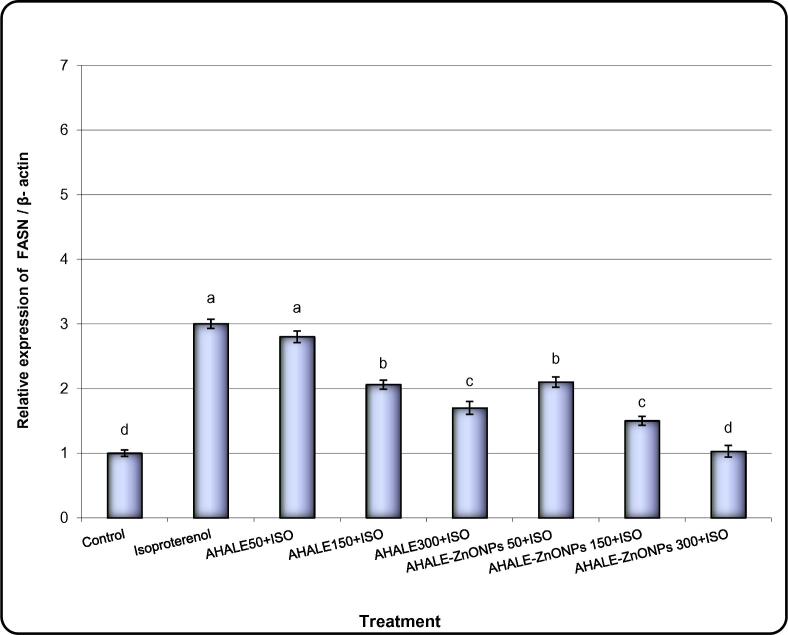
Levels of expression of PPAR-α, ADD1, FASN, and ACC genes in the heart tissues of animals treated with ISO were significantly (P < 0.05) increased in comparison with normal animals (Fig. 4, Fig. 5, Fig. 6, Fig. 7). Nonetheless, the expression levels of ADD1, FASN, and ACC genes were considerably decreased in rats pretreated with AHALE (at medium and high doses) and AHALE–ZnONPs (at all three doses) followed by exposure to ISO compared to those exposed to ISO only (Fig. 7, Fig. 8, Fig. 9, Fig. 10).
Fig. 6.
Effect of AHALE and AHALE-ZnONPs on the changes in intracellular ROS generation levels in MI induced rats. Results are expressed as the mean ± SD. a,b,c,d,e Mean with different letters, within tissue, differs significantly (p < 0.05).
Fig. 7.
Alteration of PPAR-a gene expression in heart samples of MI induced rats treated with AHALE and AHALE-ZnONPs. Data are presented as mean ± SEM. a,b,c,d,e Mean values within tissue with different Scheme 0.
Fig. 8.
Alteration of ADD1 gene expression in heart samples of MI induced rats treated with AHALE and AHALE-ZnONPs. Data are presented as mean ± SEM. a,b,c,d,e Mean values within tissue with different Scheme 0.
Fig. 9.
Alteration of FASN gene expression in heart samples of MI induced rats treated with AHALE and AHALE-ZnONPs. Data are presented as mean ± SEM. a,b,c,d,e Mean values within tissue with different Scheme 0.
Fig. 10.
Alteration of ACC gene expression in heart samples of MI induced rats treated with AHALE and AHALE-ZnONPs. Data are presented as mean ± SEM. a,b,c,d,e Mean values within tissue with different superscript letters were significantly different (p < 0.05).
4. Discussion
The results of the current study showed an increase in the concentration of CK, CK-MB, AST, ALT, and LDH enzymes in the rats treated with ISO, which are clear signs of the extent of myocardial infarction. When the heart is not supplied with glucose or oxygen, the heart muscle cells are damaged, and this may lead to the rupture of the heart membrane, which becomes permeable. This leads to leakage of the heart enzymes into the blood. When the infusion of the enzymes continues for a long time, their concentrations in the serum become high (Mathew et al., 1985, Scully et al., 2017). It was found that pretreatment of rats with AHALE and AHALE–ZnONPs for four weeks led to a reduction in the enzyme activity in the serum of animals exposed to ISO later. This shows the protective effect of AHALE and AHALE–ZnONPs on the heart muscle that was exposed to the ISO and thus reduced the damage to the heart muscle and decreased the leakage of these enzymes into the bloodstream.
The increase in the levels of TG, TC, LDL, and troponin as well as a decline in the levels of HDL in rats exposed to ISO in this study elevate the risk of myocardial infarction and of subsequent cardiac death.
The results of the current study are in agreement with those published by Acikel et al. (2005). All tissues associated with the metabolism of glycolysis contained the LDH enzyme. This enzyme has several different isoforms, ranging from LDH-1 to LDH-5. Two types LDH-1 and LDH-2 were prevalent in the heart tissues. Therefore, when heart tissue damage occurred, the concentration of LDH-1 and LDH-2 in the bloodstream elevated as a result of increased heart damage. Thus, the detection of this enzyme is considered as one of the clear diagnostic clues for heart disorders. Moreover, one reason that explains the importance of analyzing LDH enzymes is that these enzymes increase their concentration within a few hours after myocardial infarction (estimated from 12 to 24 h) and reach their peak after two to three days and start in decline after 5–14 days (Jaffe et al., 1996, Tran et al., 2015). It was also found that the increase in the levels of this enzyme in the heart tissues of animals exposed to ISO is an indicator for an increase in necrosis and apoptosis in the myocardium (Priscilla and Prince, 2009). The current results also found animals treated with ISO exhibited high levels of apoptosis and necrosis when compared to normal animals.
This study found that pretreatment of animals with AHALE and AHALE–ZnONPs for several weeks and exposed later to ISO decreased their levels of serum LDH enzyme and cTnT compared to rats exposed to ISO only. This protective action of Artemisia herba alba extract against ISO may be due to its ability to reduce the degree of damage to the heart muscle and thus prevent the outflow of these enzymes into the blood serum. These findings are consistent with the reported results of Abdallah et al. (2019), who found that pretreatment of rats with Artemisia herba alba extract improved serum markers of cardiotoxicity, prevented oxidative stress, and reduced cardiac abnormalities induced by chemotherapy.
Recently, many scientists have shown great interest in the study of apoptosis and free radical formation in the cells, which may show marked modulation in biological molecules resulting in various cases of diseases (Priscilla and Prince, 2009). Thus, the use of natural antioxidants may play an important role in reducing ROS and apoptosis in different tissues (Elhinnawi et al., 2018). In this study, we analyzed lipid peroxide and its ability to cause damage to the myocardial membranes. The results showed that exposing rats to ISO induced an elevation in TBARS and LOOH levels in cardiac tissues. The increase in these compounds may lead to damage in the heart muscle. The current study showed that pretreatment of rats with AHALE and AHALE–ZnONPs prior to exposure to ISO decreased the TBARS and LOOH levels in cardiac tissues, which were induced by ISO. Based on the fact that antioxidant compounds are playing an important role against ISO in reducing ROS generation and apoptosis (Sekiou et al., 2020), the current study evaluated the potential impact of AHALE and AHALE–ZnONPs to mitigate apoptosis and ROS generation as well as DNA damage in heart tissues These results proved that the antioxidants in AHALE and AHALE–ZnONPs may inhibit the ROS generation by ISO [38]. Therefore, we have assessed in the current study the ability of AHALE and AHALE–ZnONPs to reduce apoptosis and ROS generation as well as DNA damage in heart tissues. The results found that pretreatment of male rats with AHALE and AHALE–ZnONPs and then exposed to ISO exhibited low levels of apoptosis, ROS generation, and DNA damage compared to those in rats exposed to ISO only. Similarly, Mojarrab et al. (2016) found that Artemisia extract exhibits protective effect against DOX-induced apoptosis and DNA damage in vitro. They explained that the protecting actions of the Artemisia extract could be due to the presence of antioxidants reducing ROS formation.
Antioxidants are the first line of defense that reduces the formation of free radicals and thus reduces associated cellular toxicity. It is therefore important to use natural antioxidants to balance the antioxidants and the presence of free radicals in cellular systems to reduce or eliminate intracellular oxidative stress. In pathological conditions such as MI, this balance is usually disturbed as a result of increased production of ROS within cells under the influence of the toxicity caused by the ISO treatment. Antioxidant enzymes such as GPx, GRx, and GST, which act as free radical scavengers are the first step of protection for cells from oxidative damage (Khalil et al., 2019).
The results of this study showed that the GPx and GST activities were decreased in the rats exposed to ISO in comparison with normal animals, which may be due to a reduction in the glutathione levels in the heart tissues. It has been reported that the lack of GRx enzyme activity in heart tissue leads to the gathering of harmful glutathione, namely GSSG (oxidized glutathione) (Ferrari et al., 1985). Increasing the level of GSSG in the cell suppresses the function of enzymes having an SH group, which prevents protein production (Ji et al., 1988). However, the GPx, GRx, and GST activities were improved in animals pretreated with AHALE and AHALE–ZnONPs followed by ISO exposure compared with rats exposed to ISO only. This protective role of AHALE and AHALE–ZnONPs explains the antioxidant capacity of Artemisia extract against ROS induced heart injury.
Expression of regulator genes for lipid metabolism such as PPAR-α, ADD1, FASN, and ACC genes were assessed to understand the protective role of Artemisia extract against ISO induced hyperlipidemia (Sasikumar and Devi, 2001). Expression levels of PPAR-α, ADD1, FASN, and ACC genes in heart samples of animals treated with ISO were upregulated in comparison with those in normal rats. Nonetheless, these genes were downregulated considerably in animals pretreated with AHALE and AHALE–ZnONPs followed by exposure to ISO compared to those exposed to ISO only.
Feeding rats a diet rich in cholesterol and then injecting them with isoproterenol in the current study led to an increase in lipid markers in the lipid profile. These results are in line with the results of numerous reports that show increased levels of TC, TG, and LDL-c in the blood serum (Nagoor Meeran et al., 2012, Abo-Gresha et al., 2014, Mahmoud et al., 2014), which increase the potential for atherosclerosis and the subsequent occurrence of heart disease (Ferdinandy et al., 2007). It is also clear that the increase in the levels of lipid markers is correlated with the increase in the levels of the expression of PPAR-α, ADD1, FASN, and ACC genes. On the other hand, it was found that pretreatment with AHALE and AHALE–ZnONPs reduced the levels of lipid markers and decreased the expression levels of the lipid metabolism related genes. These findings suggested that the hypolipidemic activity of Artemisia extract could be attributed to the existence of phenolic compounds and flavonoids (El-Tantawy, 2015), which are considered to be physically powerful antioxidants reducing lipid profile in rats exposed to ISO.
5. Conclusion
Based on the findings obtained from this study, which exhibit the ability of AHALE or AHALE–ZnONPs to regulate lipid markers and genes responsible for lipid metabolism, as well as its ability to reduce oxidative stress (decrease the ROS generation, DNA damage, and apoptosis), it could be considered as one of the natural compounds that can be used for prevention of atherosclerosis and heart diseases. The reduced oxidative stress of Artemisia extract may be a result of the existence of antioxidants such as flavonoids and phenolic compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah H.M.I., Abdel-Rahman R.F., El Awdan S.A., Allam R.M., El-Mosallamy A.E.M.K., Selim M.S., Mohamed S.S., Arbid M.S., Farrag A.R.H. Protective effect of some natural products against chemotherapy-induced toxicity in rats. Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Gresha N.M., Abel-Aziz E.Z., Greish S.M. Evening primrose oil ameliorates platelet aggregation and improves cardiac recovery in myocardial-infarct hypercholesterolemic rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2014;6:23–36. [PMC free article] [PubMed] [Google Scholar]
- Acikel M., Buyukokuroglu M.E., Erdogan F., Aksoy H., Bozkurt E., Senocak H. Protective effects of dantrolene against myocardial injury induced by isoproterenol in rats: biochemical and histological findings. Intl. J. Cardiol. 2005;98:389–394. doi: 10.1016/j.ijcard.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Agate G., Stefano G., Biricolti S., Tattini M. Mesophyll distribution of ‘antioxidant’flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009;104(5):853–861. doi: 10.1093/aob/mcp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Ibrahemi N., Hasan R.M., Alslman K. Effect of zinc oxide nanoparticles on the oxidative stress (Malonaldehyde MDA, Lipid Peroxidation Level LPO) and antioxidants (GSH glutation) Medico-legal Update. 2020;20(1):882–888. [Google Scholar]
- Alshehri M.A., Aziz A.T., Trivedi S., Alanazi N.A., Panneerselvam C., Baeshen R., Alatawi A. One-step synthesis of Ag nanoparticles using aqueous extracts from sundarbans mangroves revealed high toxicity on major mosquito vectors and microbial pathogens. J. Cluster Sci. 2019;31:177–184. [Google Scholar]
- Anversa P., Sonnenblick E.H. Ischemic cardiomyopathy: pathophysiological mechanisms. Prog. Cardiovasc. Dis. 1990;33:49–70. doi: 10.1016/0033-0620(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Argolo A.C., Sant’Ana A.E., Pletsch M., Coelho L.C. Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour. Technol. 2004;95:229–233. doi: 10.1016/j.biortech.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Atale N., Saxena S., Nirmala J.G., Narendhirakannan R., Mohanty S., Rani V.J. Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: a green approach. Appl. Biochem. Biotechnol. 2017;181:1140–1154. doi: 10.1007/s12010-016-2274-6. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A.J., Beindroff C.M., Koster J.F. Superoxide dependent and independent mechanisms of iron mobilization from ferritin by xanthine oxidase; implications for oxygen radical induced tissue, destruction during ischemia and inflammation. Biochem. J. 1986;239:169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J., Arabski M., Krupa R., Wozniak K., Zadrozny M., Kasznicki M., Zurawska M., Drzewoski J. DNA damage and repair in type 2 diabetes mellitus. Mutat. Mutat. Res. 2004;554(1–2):297–304. doi: 10.1016/j.mrfmmm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P., Schulz R., Baxter G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- Ferrari R., Ceconi C., Curello S., Guarnieri C., Calderera C.M., Albertini A., Visioli O. Oxygen mediated myocardial damage during ischemia and reperfusion; role of cellular defenses against oxygen toxicity. J. Mol. Cell. Cardiol. 1985;17:937–945. doi: 10.1016/s0022-2828(85)80074-2. [DOI] [PubMed] [Google Scholar]
- Elhinnawi M.A., Mohareb R.M., Rady H.M., Khalil W.K.B., Abd Elhalim M.M., Elmegeed G.A. Novel pregnenolone derivatives modulate apoptosis via Bcl-2 family genes in hepatocellular carcinoma in vitro. J. Steroid Biochem. Mol. Biol. 2018;183:125–136. doi: 10.1016/j.jsbmb.2018.06.006. [DOI] [PubMed] [Google Scholar]
- El-Tantawy W.H. Biochemical effects, hypolipidemic and anti_inflammatory activities of Artemisia vulgaris extract in hypercholesterolemic rats. J. Clin. Biochem. Nutr. 2015;57(1):33–38. doi: 10.3164/jcbn.14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C.G., Leibovitz B.E., Tappel A.L. Lipid peroxidation measured as thiobarbituric acid reactive substances in tissue slices; characterization and comparison with homogenate and microsomes. Free Radic. Biol. Med. 1988;4:155–161. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Jakoby W.B. Assays for differentiation of glutathione-Stransferases. Meth. Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Harrison D., Griendling K.K., Landmesser U., Horing B., Dexler H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003;91:7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- Horn H.D., Burns G.H. In: Methods of Enzymatic Analysis. Bergmeyer H.V., editor. Academic Press; New York: 1978. Assay of glutathione reductase activity; p. 142. [Google Scholar]
- Iqbal R., Akhtar M.S., Hassan M.Q., Jairajpuri Z., Akhtar M., Najmi A.K. Pitavastatin ameliorates myocardial damage by preventing inflammation and collagen deposition via reduced free radical generation in isoproterenol-induced cardiomyopathy. Clin. Exp. Hypertens. 2019;41(5):434–443. doi: 10.1080/10641963.2018.1501059. [DOI] [PubMed] [Google Scholar]
- Jaffe A.S., Landt Y., Parvin C.A., Abendsehein D.R., Geltman E.M., Ladenson J.H. Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction. Clin. Chem. 1996;42:1770–1776. [PubMed] [Google Scholar]
- Jiang Z.Y., Hunt J.V., Wolff S.P. Ferrous iron oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low-density lipoprotein. Anal. Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Ji L.L., Stratman F.W., Lardy H.A. Antioxidant enzyme systems in rat liver and skeletal muscle: influences of selenium deficiency, chronic training, and acute exercise. Arch. Biochem. Biophys. 1988;263:150–160. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- Khalil W.K.B., Ahmed E.S., Kassem S.M., Shoman T.M.T., Hassanane M.M., Eshak M.G. Antioxidant capacity of Nitraria retusa leaf extracts against mitomycin C-induced genetic toxicity in male mice. J. Basic Appl. Zool. 2019;80:26. [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katus H.A., Remppis A., Scheffold T., Diederich K.W., Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am. J. Cardiol. 1991;67:1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang M., Liang Y., Wang C., Naruse K., Takahashi K. Treatment of oxidative stress with exosomes in myocardial ischemia. Int. J. Mol. Sci. 2021;22(4):1729. doi: 10.3390/ijms22041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud H.M., Zaki H.F., El Sherbiny G.A., Abd El-Latif H.A. Modulatory role of chelating agents in diet-induced hypercholesterolemia in rats. Bull. Fac. Pharm. Cairo Univ. 2014;52:27–35. [Google Scholar]
- Mair J. Progress inmyocardial damage detection: newbiochemical markers for clinicians. Crit. Rev. Clin. Lab. Sci. 1997;34:1–66. doi: 10.3109/10408369709038215. [DOI] [PubMed] [Google Scholar]
- Mathew S., Menon P.V., Kurup P.A. Effect of administration of vitamin A, ascorbic acid and nicotinamide adenine dinucleotide and flavine adenine nucleotide on severity ofmyocardial infarction induced by isoproterenol in rats. Ind. J. Exp. Biol. 1985;23:500–504. [PubMed] [Google Scholar]
- Mojarrab M., Mehrabi M., Ahmadi F., Hosseinzadeh L. Protective effects of fractions from Artemisia biennis hydro-ethanolic extract against doxorubicin-induced oxidative stress and apoptosis in PC12 cells. Iran J. Basic Med. Sci. 2016;19(5):503–510. [PMC free article] [PubMed] [Google Scholar]
- Nagoor Meeran M.F., Stanely Mainzen Prince P., Hidhayath Basha R. Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study. Eur. J. Pharmacol. 2012;677:116–122. doi: 10.1016/j.ejphar.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Neha A., Vibha R., Sciences B. GC-MS analysis of bioactive components in the ethanolic and methanolic extract of Syzygium cumini. Int. J. Pharma Bio Sci. 2013;4:296–304. [Google Scholar]
- Prasannaraj G., Venkatachalam P. Hepatoprotective effect of engineered Zinc Oxide nanoparticles coated bioactive compounds against diethylnitrosamine induced hepatocarcinogenesis in experimental mice. J. Photochem. Photobiol. B. 2017;167:309–320. doi: 10.1016/j.jphotobiol.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Priscilla D.H., Prince P.S.M. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Radulović N.S., Randjelović P.J., Stojanović N.M., Blagojević P.D., Stojanović-Radić Z.Z., Ilić I.R., Djordjević V.B. Toxic essential oils. Part II: chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013;58:37–49. doi: 10.1016/j.fct.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Román G.C., Jackson R.E., Gadhia R., Román A.N., Reis J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. (Paris). 2019;175(10):724–741. doi: 10.1016/j.neurol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstraw W.G. Selenium; biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Salem N.A., Wahba M.A., Eisa W.H., El-Shamarka M., Khalil W. Zinc oxide nanoparticles alleviate indomethacin-induced gastric injury: a novel antiulcer agent. Inflammopharmacology. 2018;26(4):1025–1035. doi: 10.1007/s10787-017-0424-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C., Larrauri J.A., Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998;76:270–276. [Google Scholar]
- Sandoval Y., Jaffe A.S. Type 2 myocardial infarction: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;73(14):1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Sasikumar S.C., Devi S.C.S. Effect of ‘abana’ pretreatment on isoproterenol-induced hyperlipidemia in rats. Indian J. Pharm. Sci. 2001;63(2):101–104. [Google Scholar]
- Scully M., Knöbl P., Kentouche K., Rice L., Windyga J., Schneppenheim R., Kremer Hovinga J.A., Kajiwara M., Fujimura Y., Maggiore C., Doralt J., Hibbard C., Martell L., Ewenstein B. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017;130(19):2055–2063. doi: 10.1182/blood-2017-06-788026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiou O., Boumendjel M., Taibi F., Tichati L., Boumendjel A., Messarah M. Nephroprotective effect of Artemisia herba alba aqueous extract in alloxan-induced diabetic rats. J. Tradit. Complement. Med. 2020;11(1):53–61. doi: 10.1016/j.jtcme.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalyapina, A.Y., Solovyova, A.Y., Zaporo zhets, M.A., Khokhlov, E.M., Plotnichenko, V.G., Buslae va, E.Yu., Rustamova, E.G., Gubin, S.P., 2012, Composite materi als based on graphene and zinc oxide nanoparticles Bulle9tin of MITXT. 7 (5), 80984.
- Skowyra M., Gallego M.G., Segovia F., Almajano M.P. Antioxidant properties of artemisia annua extracts in model food emulsions. Antioxidants. 2014;3:116–128. doi: 10.3390/antiox3010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran B.H., Huang C., Zhang Q., Liu X., Lin S., Liu H., Wang S., Zhu Y.Z. Cardioprotective effects and pharmacokinetic properties of a controlled release formulation of a novel hydrogen sulfide donor in rats with acute myocardial infarction. Biosci. Rep. 2015;35(3) doi: 10.1042/BSR20140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M., Martinez-Serrano A., Borner C., Blanco P., Satrustegui J. NMDA-induced increase in [Ca2+](i) and 45Ca2+ uptake in acutely dissociated brain cells derived from adult rats. Brain Res. 1992;570(1–2):347–353. doi: 10.1016/0006-8993(92)90600-e. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Wang L.F. Theoretical elucidation on structure-antioxidant activity relationships for indolinic hydroxylamines. Bioorg. Med. Chem. Lett. 2002;12:229–233. doi: 10.1016/s0960-894x(01)00724-7. [DOI] [PubMed] [Google Scholar]