Abstract
Background:
It remains debated whether any single coronary atherosclerotic plaque within the vulnerable patient exhibits unique morphology conferring an increased risk of clinical events.
Objectives:
To precisely phenotype culprit and nonculprit lesions in myocardial infarction (MI) and lesions in stable coronary artery disease (CAD) using coronary computed tomography angiography (CCTA)-based radiomic analysis.
Methods:
Sixty patients with acute MI prospectively underwent CCTA prior to invasive angiography and were matched to 60 patients with stable CAD. For all coronary lesions, high-risk plaque (HRP) characteristics were qualitatively assessed, followed by semi-automated plaque quantification and extraction of 1,103 radiomic features. Machine learning models were built to assess the additive value of radiomic features for discriminating culprit lesions over and above HRP and plaque volumes.
Results:
Culprit lesions had higher mean volumes of noncalcified plaque (NCP) and low-density noncalcified plaque (LDNCP) compared with the highest-grade stenosis nonculprits and highest-grade stenosis stable CAD lesions (NCP: 138.1mm3 vs. 110.7mm3 vs. 102.7mm3; LDNCP: 14.2mm3 vs. 9.8mm3 vs. 8.4mm3; both ptrend<0.01). In multivariable linear regression adjusted for NCP and LDNCP volumes, 14.9% (164/1,103) of radiomic features were associated with culprits and 9.7% (107/1,103) were associated with the highest-grade stenosis nonculprits (critical p<0.0007) when compared with highest-grade stenosis stable CAD lesions as reference. Hierarchical clustering of significant radiomic features identified 9 unique data clusters (latent phenotypes): 5 contained radiomic features specific to culprits, 1 contained features specific to highest-grade stenosis nonculprits, and 3 contained features associated with either lesion type. Radiomic features provided incremental value for discriminating culprit lesions when added to a machine learning model containing HRP and plaque volumes (area under the receiver operating characteristic curve 0.86 vs. 0.76, p=0.004).
Conclusions:
Culprit lesions and highest-grade stenosis nonculprit lesions in MI have distinct radiomic signatures. Within the vulnerable patient may exist individual vulnerable plaques identifiable by CCTA-based precision phenotyping.
Keywords: coronary computed tomography angiography, coronary plaque, myocardial infarction, radiomics, machine learning
INTRODUCTION
The concept of the “vulnerable patient” is well-established, with coronary atherosclerotic disease burden being a strong predictor of subsequent acute coronary syndrome (ACS) in individuals with stable coronary artery disease (CAD) (1). However, it is debated whether any single “vulnerable plaque” in these patients exhibits unique morphological features conferring an increased risk of clinical events over total atheroma burden (1,2). Intracoronary imaging studies of culprit lesions at the time of ACS have identified vulnerable plaque features such as a large lipid-rich necrotic core, thin fibrous cap, and microcalcifications (3). Yet, invasive plaque characterization remains technically difficult and yields a low positive predictive value for future ACS (4). Coronary computed tomography angiography (CCTA) is a noninvasive first-line modality for the evaluation of suspected CAD (5). Qualitative plaque characteristics and quantitative attenuation-based plaque measures on CCTA have demonstrated prognostic value for patient-level risk of coronary events (6–8). Beyond these conventional parameters, however, may exist latent morphological plaque features which are not captured by current CCTA analysis techniques. Radiomics, by extracting thousands of computational quantitative features from medical images, enables precision phenotyping of diseases in vivo (9). Prior reports have shown CCTA-based radiomic analysis to reliably detect vulnerable plaques in patients with stable CAD when compared to invasive and histological reference standards (10,11). This study aimed to evaluate whether culprit lesions in acute myocardial infarction (MI) have a distinct radiomic signature on CCTA compared with nonculprit lesions in the same patients and with lesions in stable CAD patients. We also sought to determine the incremental value of radiomic features for discriminating culprit lesions beyond current state-of-the-art plaque assessment in CCTA.
METHODS
Study Population
This was a post-hoc analysis of the prospective INFLAME study (Inflammation of pericoroNary Fat and its association with coronary atheroscLerosis Assessed by coMputEd tomography coronary angiography; Australian New Zealand Clinical Trials Registration ACTRN12618001058268). The patient selection and study design have been previously published (12). Briefly, 60 consecutive patients presenting with acute MI at MonashHeart, Melbourne, Australia were recruited to undergo CCTA within 48 hours of admission, prior to invasive coronary angiography (ICA). These patients were matched to 60 outpatients with stable CAD (defined by stable exertional symptoms and/or inducible myocardial ischemia on stress testing) who underwent CCTA during the same time period. Matching was performed for age, sex, body mass index (BMI), risk factors (diabetes, hypertension, dyslipidemia, smoking), medications, and CT tube voltage (Supplemental Appendix). The study was approved by the local Human Research Ethics Committee (Monash Health HREC 15244A) and all MI patients provided written informed consent.
MI and Culprit Lesion Adjudication
Adjudication of MI was performed by a panel of 2 cardiologists (A.L. and D.W.) using the Fourth Universal Definition (13). Cases were classified as ST-segment elevation MI (STEMI) or non-ST-segment elevation MI (NSTEMI). One culprit lesion per patient was identified at the time of ICA by an experienced interventional cardiologist (the primary operator) blinded to CCTA findings, using a combination of angiographic appearance (stenosis severity and lesion morphology), electrocardiogram findings, and wall motion abnormalities on left ventriculography or echocardiography. Independent adjudication of culprit lesions was performed post-hoc by a second interventional cardiologist (D.W.) blinded to the decisions of the primary operator. Ambiguous cases or cases with multiple candidate culprits were resolved by consensus. All other lesions were deemed nonculprit lesions.
Ascertainment of Risk Factors
Pre-existing cardiovascular risk factors and medications were documented on admission for the MI cohort and ascertained by review of electronic medical records for the stable CAD cohort. Risk factors are defined in the Supplemental Appendix.
CCTA Acquisition
All scans were performed on a 320-detector-row CT scanner (Aquilion ONE ViSION, Canon Medical Systems, Otawara, Japan) as previously described (14) (Supplemental Appendix).
Qualitative Plaque Analysis
CCTA analysis was performed at the Cedars-Sinai Medical Center core laboratory by a level III-experienced reader (A.L.) blinded to clinical data and ICA findings. Using axial and multiplanar reconstruction views, all coronary segments ≥2 mm were evaluated according to an 18-segment model (15). For each patient, we calculated the segment involvement score and segment stenosis score (16) as qualitative measures of the extent and severity of CAD. Each coronary lesion was assessed for adverse plaque characteristics (6,17) (APCs; Supplemental Appendix). High-risk plaque (HRP) was defined by the presence of ≥2 APCs including positive remodeling, low attenuation plaque, spotty calcification, or napkin-ring sign. A second expert reader (D.H.) in the core laboratory independently assessed all lesions for the presence of HRP; interobserver agreement was moderate (κ=0.58).
Quantitative Plaque Analysis
Standardized plaque measurements were performed using semiautomated research software (Autoplaque v2.5, Cedars-Sinai Medical Center, Los Angeles, CA, USA) (18,19) (Supplemental Appendix). Plaque volume (mm3) was calculated on a per-lesion level for the following plaque components: total plaque (TP), calcified plaque (CP), noncalcified plaque (NCP), and low-density noncalcified plaque (LDNCP; defined by an attenuation of <30 Hounsfield units [HU]). The respective plaque burdens (%) were calculated as: plaque volume / analyzed vessel volume × 100 (20). Plaque volume and burden measurements for the entire coronary tree were summarized on a per-patient level. Per-lesion plaque composition (%) by CP, NCP, and LDNCP components was calculated as: plaque component volume / total plaque volume × 100 (19). Additionally, for each lesion, the software automatically quantified plaque length, percent diameter stenosis, and vessel remodeling index (Supplemental Appendix). Stenosis severity was further categorized according to the Coronary Artery Disease Reporting and Data System (CAD-RADS): 0 (0%), 1 (1–24%), 2 (25–49%), 3 (50–69%), 4 (70–99%), 5 (100%) (21). Following CCTA plaque analysis, ICA-determined culprit lesions were co-registered to lesions on CCTA (Supplemental Appendix).
Radiomic Plaque Analysis
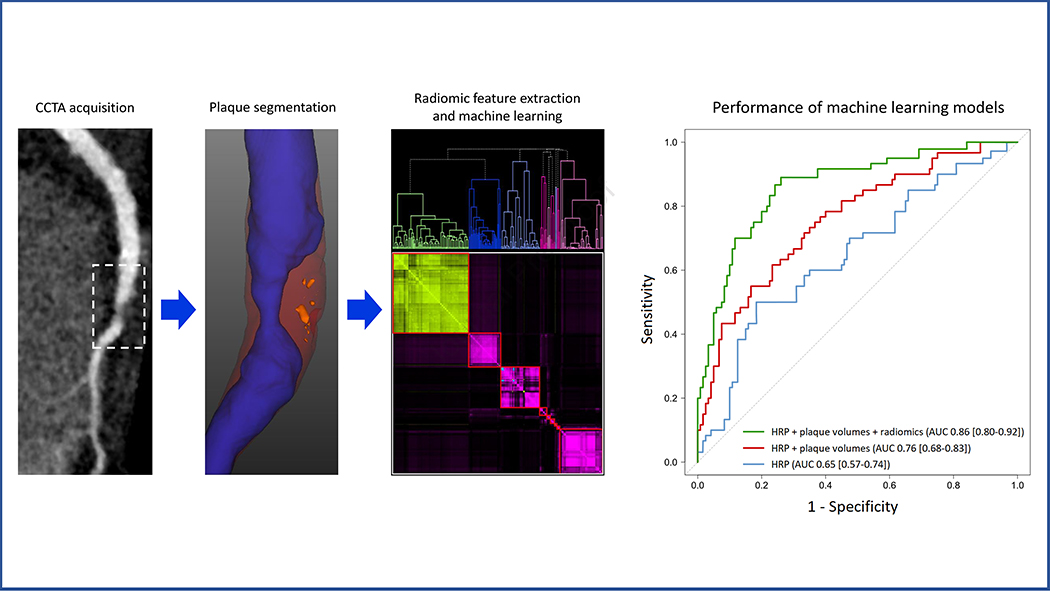
All voxels defined as plaque by the semiautomated software (Autoplaque v2.5) were extracted as DICOM images and loaded into the open-source Radiomics Image Analysis (RIA v1.4.2) software package in the R environment (22). This process was performed for: 1) culprit lesions; 2) all nonculprit lesions as one segmentation; 3) all lesions in stable CAD as one segmentation; 4) highest-grade stenosis non-culprit lesions; 5) highest-grade stenosis lesions in stable CAD. The images were discretized to 8, 16, and 32 equally sized bins with identical HU ranges, creating 3 replicas of each image. A total of 1,103 radiomic features were calculated for each segmentation, including: 44 first-order metrics describing the distribution of HU values; 342 gray-level co-occurrence matrix (GLCM) textural parameters describing how often voxels of a similar value occur next to each other; 33 gray-level run-length matrix (GLRLM) textural parameters describing how often a given number of similar-value voxels occur next to each other; and 684 geometric parameters describing spatial characteristics such as shape, size, or volume. A detailed description of each radiomic feature has been previously published (23–25). Central Illustration shows the workflow for plaque segmentation and radiomic analysis.
Central Illustration. Radiomics-based precision phenotyping in myocardial infarction.
Plaque segmentation was performed on CCTA images (case example of a culprit lesion shown) using semiautomated software. Noncalcified plaque is displayed in red, low-density noncalcified plaque in orange, and vessel lumen in blue. Radiomic features were extracted from plaque-containing voxels and analyzed using hierarchical clustering. Nested machine learning models were built using conventional plaque parameters and cluster-derived radiomic features; the performance of these models in discriminating culprit lesions is shown on the right. AUC = area under the receiver operating characteristic curve; HRP = high-risk plaque.
Machine Learning
Model Building and Training
Given the high dimensionality of our dataset and the complex inter-relationships that exist between plaque features, we applied machine learning (ML) to our radiomic analysis; an approach previously shown to be useful for identifying advanced atherosclerotic lesions (10). To examine the incremental value of radiomic features beyond conventional plaque analysis for discriminating culprit lesions from the highest-grade stenosis nonculprit and stable CAD lesions, we built 3 nested ML models using CCTA-based plaque parameters: model 1: presence of HRP; model 2: model 1 plus total plaque volume plus volumes and compositions of NCP and LDNCP; and model 3: model 2 plus 18 radiomic features with the smallest p-values from the 9 distinct data clusters (see Statistical Analysis): 9 features associated with culprits and 9 features associated with the highest-grade stenosis nonculprits. To examine the incremental value of radiomic features for culprit lesion discrimination beyond stenosis, we performed an additional ML analysis with the following parameters: model 1: stenosis severity by CAD-RADS category; model 2: model 1 plus the 18 radiomics features. We employed supervised ML with XGBoost, a state-of-the-art boosted ensemble algorithm which has demonstrated high performance within the domains of computer science and medicine (26). XGBoost uses an ensemble of gradient-boosted decision trees, combining multiple weak classifiers (one-level decision trees) to produce a single strong classifier, which can improve prediction modeling. We trained and tested all 3 of our ML models for a binary classification task (culprit lesion vs. other lesion types) using 10 times repeated 10-fold cross-validation (27) (Supplemental Appendix). All ML analyses were performed using Python 3.8.5, scikit-learn 0.23.2, and XGBoost 1.3.3.
External Validation
We evaluated the performance of the radiomics-based ML model in an external validation cohort from University Hospital Erlangen, Erlangen, Germany. This consisted of 19 patients admitted with ACS who underwent CCTA prior to ICA, as well as 16 matched controls with stable CAD (28). The patient selection and CCTA imaging protocol are detailed in the Supplemental Appendix. Clinical and CCTA characteristics of this cohort have been previously published (28). Qualitative, quantitative, and radiomic plaque analysis were performed using the same methodology described in the present study.
Statistical Analysis
At the patient level, MI patients were compared 1:1 to matched patients with stable CAD. At the lesion level, culprit lesions were compared to: 1) the average of all nonculprit lesions (within-subject) and the average of all stable CAD lesions (between-subject); and 2) the highest-grade stenosis nonculprit lesion (within-subject) and the highest-grade stenosis stable CAD lesion (between-subject), as determined by quantitative plaque analysis. We specifically examined highest-grade stenosis lesions given that coronary lumen stenosis remains the dominant measure of CAD severity used in clinical practice to guide patient management.
Continuous variables are presented as mean ± standard deviation or median (interquartile range), as appropriate. Categorical variables are expressed as frequencies (%). Comparisons of continuous variables were performed using an independent samples t-test or a one-way ANOVA with Tukey’s post-hoc test. Categorical variables were compared with a Fisher’s exact or Chi-square test. Interobserver agreement was calculated using the κ statistic. For clinical and conventional plaque parameters, a 2-sided p-value <0.05 indicated statistical significance.
For lesion-level radiomic characterization, principal component analysis identified 72 unique radiomic features which accounted for 99.5% of the variance in the data (29). Hence, applying Bonferroni correction, p-values <0.0007 (0.05/72) were considered statistically significant. To examine the association of culprit and nonculprit lesions with each of the 1,103 radiomic features, we performed linear regression analysis using lesion types as categorical predictors with stable CAD lesions as reference. We then adjusted for volumes of CP, NCP, and LDNCP to correct for any potential intrinsic correlation between plaque volume and morphology. From the multivariable linear regression, we identified the significant radiomic features associated with either culprit or nonculprit lesions and performed pairwise linear regression between these features. The resultant intra-pair R2 values were used as distance measures for hierarchical clustering. The elbow method based on the total within-cluster sum of squares was used to calculate the optimal number of distinct data clusters (latent phenotypes): 15 for culprit vs. all nonculprits; 9 for culprit vs. highest-grade stenosis nonculprit.
For clinical intuition, we performed conventional statistical logistic regression on the same models that were used in the ML analysis: model 1: HRP; model 2: model 1 plus quantitative plaque parameters; and model 3: model 2 plus radiomic features. All 3 logistic regression models were 10-fold cross-validated with the same data folds used in ML. To avoid including highly correlated radiomic features in statistical model 3, we performed backward stepwise logistic regression at a Wald p-value of 0.10, resulting in 8 radiomic features being retained in the final model. Receiver-operating characteristic analysis was used to evaluate the discriminatory ability of all models for identifying culprit lesions, and the area under the curve (AUC) values were compared using the method of Delong et al. (30). All statistical analyses were performed using R 3.5, R Studio 1.2.1335, and Stata 14.0 (StataCorp, College Station, TX, USA).
RESULTS
Baseline Patient Characteristics
Table 1 summarizes characteristics of the study population according to clinical presentation. The 2 cohorts were well-matched for age, sex, BMI, risk factors, medications, and CT tube voltage. Of patients with MI, 55 (91.7%) presented with NSTEMI and 5 (8.3%) with post-thrombolysis STEMI. All patients had stenting of the culprit lesion and 9 (15.0%) patients had stenting of the highest-grade stenosis nonculprit lesion during the index admission.
Table 1.
Patient characteristics according to clinical presentation
| Acute MI (n = 60) | Stable CAD (n = 60) | p value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, yrs | 59.9±11.6 | 60.2±11.3 | 0.68 |
| Body mass index, kg/m2 | 28.3±5.9 | 28.7±5.0 | 0.29 |
| Male sex | 52 (86.7) | 52 (86.7) | 1.00 |
| Hypertension | 44 (73.3) | 41 (68.3) | 0.49 |
| Diabetes | 15 (25.0) | 13 (21.7) | 0.53 |
| Dyslipidemia | 32 (53.3) | 35 (58.3) | 0.36 |
| Smoking | 24 (40.0) | 20 (33.3) | 0.21 |
| Family history of CAD | 25 (41.7) | 27 (45.0) | 0.47 |
| Baseline medications | |||
| Antiplatelet | 11 (18.3) | 10 (16.7) | 0.43 |
| Statin | 15 (25.0) | 17 (28.3) | 0.17 |
| Beta-blocker | 10 (16.7) | 12 (20.0) | 0.38 |
| ACE-I or ARB | 14 (23.3) | 14 (23.3) | 0.26 |
| CCTA acquisition parameters | |||
| Heart rate, bpm | 53.9±5.3 | 54.8±8.4 | 0.58 |
| Tube voltage | 1.00 | ||
| 100 kV | 22 (36.7) | 22 (36.7) | |
| 120 kV | 38 (63.3) | 38 (63.3) | |
| Contrast dose, mL | 77.6±11.0 | 76.2±13.9 | 0.83 |
| Radiation dose, DLP | 284.8±156.9 | 246.1±171.4 | 0.26 |
Values are expressed as n (%) or mean ± standard deviation.
Bold p values indicate statistical significance.
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; DLP = dose length product; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MI = myocardial infarction.
Qualitative Plaque Parameters
Patients with MI had a higher average number of lesions compared to patients with stable CAD (5.6±3.9 vs. 3.9±2.7, p=0.03). HRP was more frequent in MI patients than in stable CAD patients (70.0% vs. 31.7%, p<0.001), as was each component of HRP (all p<0.05; Table 2).
Table 2.
Per-patient level plaque analysis
| Acute MI (n=60) | Stable CAD (n=60) | p value | |
|---|---|---|---|
| Total number of lesions | 5.6±3.9 | 3.9±2.7 | 0.03 |
| Qualitative parameters | |||
| Segment involvement score | 5.8±3.1 | 5.1±2.5 | 0.38 |
| Segment stenosis score | 14.2±7.9 | 9.7±5.2 | 0.005 |
| High-risk plaque present | 42 (70.0) | 19 (31.7) | <0.001 |
| Positive remodeling present | 50 (83.3) | 33 (55.0) | <0.001 |
| Low attenuation plaque present | 46 (76.7) | 14 (23.3) | <0.001 |
| Spotty calcification present | 20 (33.3) | 10 (16.7) | <0.001 |
| Napkin-ring sign present | 16 (26.7) | 4 (6.7) | <0.001 |
| Quantitative parameters | |||
| Plaque volume, mm3 | |||
| Total plaque | 596.9±408.1 | 278.9±289.0 | <0.001 |
| Calcified plaque | 75.1±98.5 | 29.6±45.5 | 0.002 |
| Noncalcified plaque | 521.9±332.2 | 229.3±248.1 | <0.001 |
| Low-density noncalcified plaque | 36.8±24.5 | 17.2±21.1 | <0.001 |
| Plaque burden, % | |||
| Total plaque | 45.2±7.1 | 40.3±8.1 | 0.001 |
| Calcified plaque | 4.3±3.5 | 3.4±2.9 | 0.14 |
| Noncalcified plaque | 41.0±8.5 | 37.0±9.7 | 0.02 |
| Low-density noncalcified plaque | 3.1±1.6 | 2.7±1.5 | 0.03 |
Values are expressed as n (%) or mean ± standard deviation.
Bold p values indicate statistical significance.
CAD = coronary artery disease, MI = myocardial infarction.
Culprit lesions more often exhibited each of the 4 APCs compared to all nonculprit lesions in MI and all lesions in stable CAD (all p<0.05; Supplemental Table 1). When compared to the highest-grade stenosis nonculprit and stable CAD lesions, culprit lesions were more likely to exhibit low attenuation plaque or positive remodeling (all p<0.05; Table 3). There were no significant differences in the prevalence of APCs or HRP between nonculprit lesions and stable CAD lesions (Supplemental Table 1 and Table 3).
Table 3.
Per-lesion level plaque analysis for culprit vs. highest-grade stenosis nonculprit in MI vs highest-grade stenosis lesion in stable CAD
| Culprit lesion (n=60) | Highest stenosis nonculprit lesion (n=60) | Highest stenosis stable CAD lesion (n=60) | p value | p values for post-hoc comparisons |
|||
|---|---|---|---|---|---|---|---|
| Culprit vs. nonculprit | Culprit vs. stable | Nonculprit vs. stable | |||||
| Qualitative parameters | n (%) | n (%) | n (%) | ||||
| High-risk plaque present | 32 (53.3) | 15 (25.0) | 10 (16.7) | <0.001 | 0.005 | <0.001 | 0.86 |
| Positive remodeling present | 40 (66.7) | 28 (46.7) | 20 (33.3) | 0.001 | 0.04 | 0.001 | 0.58 |
| Low attenuation plaque present | 42 (70.0) | 9 (15.0) | 9 (15.0) | <0.001 | <0.001 | <0.001 | 1.00 |
| Spotty calcification present | 10 (16.7) | 10 (16.7) | 6 (10.0) | 0.09 | 1.00 | 0.86 | 0.86 |
| Napkin-ring sign present | 10 (16.7) | 7 (11.7) | 4 (6.7) | 0.53 | 1.00 | 0.72 | 1.00 |
| Quantitative parameters | |||||||
| Plaque volume, mm3 | |||||||
| Total plaque | 150.6±86.8 | 125.3±129.6 | 116.3±75.4 | <0.001 | <0.001 | <0.001 | 0.09 |
| Calcified plaque | 12.5±23.4 | 15.7±28.3 | 16.5±22.1 | 0.14 | 0.21 | 0.13 | 0.83 |
| Noncalcified plaque | 138.1±81.9 | 110.7±102.4 | 102.7±63.2 | 0.004 | 0.005 | 0.03 | 0.35 |
| Low-density noncalcified plaque | 14.2±10.7 | 9.8±8.6 | 8.4±7.9 | 0.001 | 0.008 | 0.001 | 0.42 |
| Plaque burden, % | |||||||
| Total plaque | 60.2±10.9 | 50.1±11.6 | 49.9±10.2 | <0.001 | <0.001 | <0.001 | 0.57 |
| Calcified plaque | 3.8±5.6 | 4.2±5.1 | 4.4±3.7 | 0.21 | 0.92 | 0.28 | 0.14 |
| Noncalcified plaque | 56.4±13.7 | 46.5±12.7 | 42.3±11.6 | <0.001 | <0.001 | <0.001 | 0.16 |
| Low-density noncalcified plaque | 6.7±5.5 | 4.2±2.7 | 3.1±2.2 | <0.001 | 0.008 | <0.001 | 0.03 |
| Plaque composition, % | |||||||
| Calcified plaque | 7.3±10.7 | 9.3±11.4 | 9.6±10.5 | 0.14 | 0.87 | 0.70 | 0.94 |
| Noncalcified plaque | 92.7±10.7 | 90.7±11.4 | 90.4±10.5 | ||||
| Low-density noncalcified plaque | 10.1±7.8 | 7.0±4.7 | 6.9±4.1 | 0.001 | 0.006 | 0.004 | 0.96 |
| Plaque length, mm | 17.3±7.6 | 19.7±13.7 | 18.5±11.1 | 0.11 | 0.19 | 0.76 | 0.85 |
| Diameter stenosis, % | 86.9±12.6 | 58.1±22.3 | 55.4±20.9 | <0.001 | <0.001 | <0.001 | 0.20 |
| Remodeling index | 1.38±0.3 | 1.25±0.2 | 1.21±0.2 | 0.17 | 0.32 | 0.15 | 0.74 |
Values are expressed as n (%) or mean ± standard deviation. Bold p values indicate statistical significance.
CAD = coronary artery disease, MI = myocardial infarction.
Quantitative Plaque Parameters
Patients with MI had greater volumes of all plaque components compared to patients with stable CAD (all p<0.05; Table 2). Burdens of TP (45.2±7.1% vs. 40.3±8.1%, p=0.001), NCP (41.0±8.5% vs. 37.0±9.7%, p=0.02), and LDNCP (3.1±1.6% vs. 2.7±1.75, p=0.03) were higher in MI versus stable CAD patients; with no significant differences in CP burden (p=0.14).
Culprit lesions had a mean TP volume of 150.6±86.8 mm3, comprising 12.5±23.4 mm3, 138.1±81.9 mm3, and 14.2±10.7 mm3 of CP, NCP, LDNCP, respectively. The volumes and burdens of TP, NCP, and LDNCP were higher in culprits compared to nonculprits and stable CAD lesions (all p<0.05; Supplemental Table 1 and Table 3); no significant differences were observed for CP (all p>0.05). LDNCP composition was greater in culprits (10.1±7.8%) compared to all nonculprits and all stable CAD lesions (5.9±3.3% and 5.8±2.7%, respectively; both p<0.05); and compared to the highest-grade stenosis nonculprits and highest-grade stable CAD lesions (7.0±4.7 and 6.9±4.1, respectively; both p<0.05). LDNCP burden was greater in the highest-grade nonculprits than in the highest-grade stable CAD lesions (4.2±2.7% vs. 3.1±2.2%, p=0.03); with no significant differences in the remaining quantitative parameters between the 2 plaque types (all p>0.05).
Radiomic Analysis
In linear regression analysis, 349 (31.6%) of 1,103 radiomic features were significantly associated with culprit lesions and 273 (24.8%) were associated with all nonculprit lesions, when compared with all stable CAD lesions as reference. Following multivariable adjustment for volumes of CP, NCP, and LDNCP, 367 (33.3%) radiomic features were associated with culprits, whereas no features were associated with nonculprits (Supplemental Figure 1).
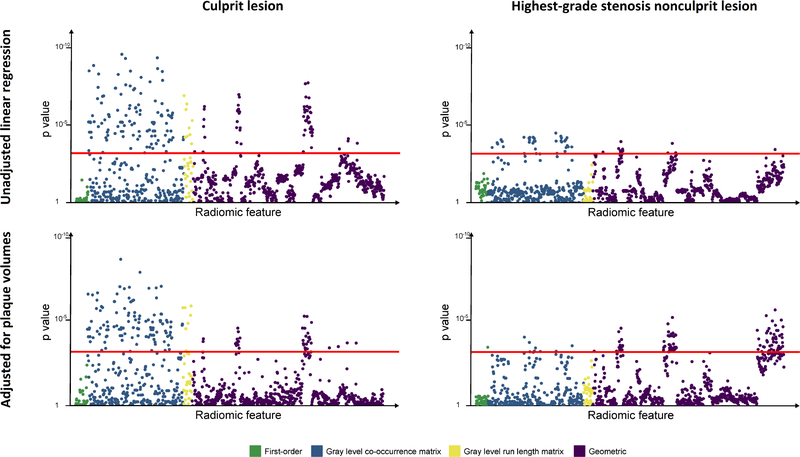
Linear regression performed using culprits and the highest-grade stenosis nonculprits showed an association with 186 (16.9%) and 47 (4.3%) radiomic features, respectively, referenced against the highest-grade stenosis stable CAD lesions. When adjusted for plaque volumes, 164 (14.9%) and 107 (9.7%) features, respectively, remained statistically significant (Figure 1). A greater proportion of textural (GLCM and GLRLM) and geometric features, rather than first-order metrics, differed between culprit and nonculprit lesions (Supplemental Table 2).
Figure 1. Manhattan plot of p-values for associations of culprit lesions and highest-grade stenosis nonculprit lesions with radiomic features.
P values are displayed on the y axis for each of the 1,103 radiomic parameters lined up on the x axis, color-coded by category. Upper panels show results from unadjusted linear regression analysis; bottom panels show results following adjustment for volumes of calcified, noncalcified, and low-density noncalcified plaque. Points above the red line (p=0.0007) indicate radiomic features significantly associated with either culprit or highest-grade stenosis nonculprit lesions. The highest-grade stenosis lesion in stable CAD was used as reference.
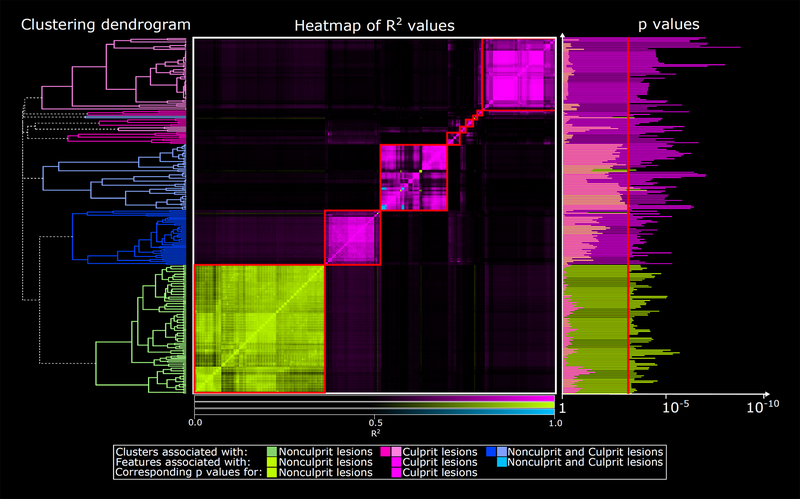
On cluster analysis of significant radiomic features, we identified 15 statistically unique data clusters (latent phenotypes); each cluster contained radiomic features associated exclusively with culprit lesions (Supplemental Figure 2). When comparing culprits to the highest-grade stenosis nonculprits, we identified 9 distinct clusters. Of these, 5 clusters contained only radiomic features specific to culprits, 1 contained only features specific to nonculprits, and 3 contained features associated with either lesion type (1 cluster contained 6 features common to both lesion types) (Figure 2).
Figure 2. Clustering dendrogram and heatmap of significant radiomic features associated with culprit vs. highest-grade stenosis nonculprit lesion.
There were 9 distinct clusters (red boxes) among the 265 significant radiomic features when comparing the culprit lesion and highest-grade stenosis nonculprit lesion (middle panel). Of these, 5 clusters contained only radiomic features specific to culprit lesions (pink), 1 cluster contained only features specific to nonculprit lesions (green), and 3 clusters contained features associated with either lesion type (1 cluster contained 6 features associated with both lesion types, as shown in blue). The highest-grade stenosis stable CAD lesion was used as reference.
Machine Learning for Identification of Culprit Lesions
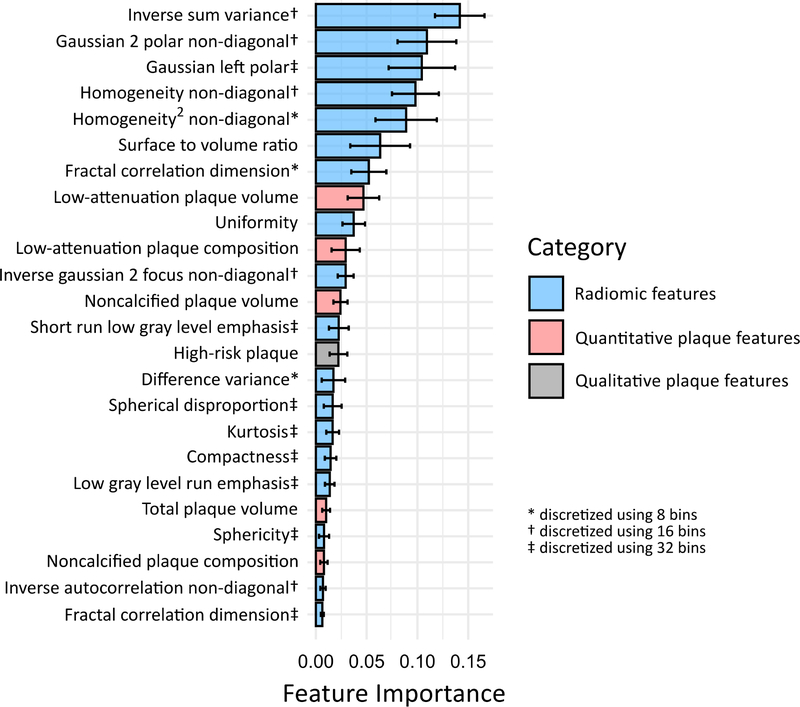
In the ML models, adding quantitative plaque parameters to HRP resulted in a significantly higher AUC for discriminating culprit lesions (0.76, 95% confidence interval [CI] 0.68–0.83) compared to HRP alone (0.65, 95% CI 0.57–0.74; p=0.01). Further addition of radiomic features provided incremental discriminatory value over and above HRP and quantitative plaque parameters (AUC 0.86, 95% CI 0.80–0.92; p=0.004; Central Illustration). The ranking of feature importance for identifying culprit lesions in the final ML model is shown in Figure 3. Textural (GLCM) and geometric radiomic features occupied the top 7 positions overall, while LDNCP volume and composition were highest-ranked among quantitative plaque parameters. When evaluated in the external validation cohort, the radiomics-based ML model exhibited an AUC of 0.84 (95% CI 0.74–0.94) for culprit lesion discrimination.
Figure 3. Feature importance for machine learning identification of culprit lesions.
Ranking of feature importance in the final ML model incorporating HRP, quantitative plaque parameters, and radiomic features. The solid bars and error bars represent the mean feature importance value and standard deviation, respectively.
ML analysis in the main cohort also showed radiomic features alone (AUC 0.84, 95% CI 0.78–0.91) to outperform the combination of HRP and quantitative plaque parameters (p=0.009) and HRP alone (p<0.001) for discriminating culprit lesions. The addition of radiomic features to a ML model with stenosis severity provided incremental value for culprit lesion discrimination, with an AUC increase from 0.83 (95% CI 0.77–0.89) to 0.89, (95% CI 0.84–0.94; p=0.002).
Performance of Conventional Statistical Models
In logistic regression analysis, a model with radiomic features, plaque volumes, and HRP (AUC 0.80, 95% CI 0.74–0.85) outperformed a model with plaque volumes and HRP (AUC 0.71, 95% CI 0.65–0.76; p=0.005) and HRP alone (AUC 0.63, 95% CI 0.57–0.69; p<0.001) for culprit lesion discrimination.
DISCUSSION
Our primary findings are: 1) culprit lesions in MI have a distinct radiomic phenotype on CCTA compared with nonculprit lesions and stable CAD lesions; 2) among all nonculprits, the highest-grade stenosis lesion carries a unique radiomic signature; 3) the distinguishing radiomic features in both scenarios are textural and geometric; and 4) radiomic features provide incremental value for discriminating culprit lesions beyond current state-of-the-art CCTA-based plaque assessment.
Radiomic analysis enables precision phenotyping of diseases in vivo, by extracting thousands of computational quantitative features from an imaging region of interest which potentially reflect the underlying pathophysiology (9). Coronary plaques comprise several different histological components which vary in their CT HU attenuation values (31). This has enabled the noninvasive assessment of plaque morphology, with numerous CCTA-derived qualitative (6,17) and quantitative (7,8) parameters associating with an increased risk of subsequent ACS. However, visual plaque assessment is prone to inter-reader variability (32), and semiautomated plaque quantification relies on absolute voxel attenuation values (31) without considering the complex spatial relationship between voxels. Radiomics provides an objective, automated, data-driven description of plaque morphology using measures of heterogeneity or shape which are mostly invisible to the human eye (24). This approach has outperformed conventional qualitative and quantitative metrics for identifying vulnerable plaques in stable patients undergoing CCTA when compared to invasive reference standards (10). Further, in an ex vivo study, radiomic features improved the accuracy of CCTA for identifying histologically-verified thin-cap fibroatheroma (11). The present analysis extends these findings by demonstrating that radiomic analysis can reliably detect and characterize MI-causing unstable plaques at the time of event.
Several prior studies have utilized CCTA for plaque assessment in patients presenting with ACS. Early reports showed a higher prevalence of qualitative APCs in culprit lesions compared to lesions in stable CAD (33,34). Subsequent investigators used software applications to quantify plaque volumes and burdens in the setting of ACS (20,35). The current study is the first to perform radiomic phenotyping of plaque in patients with acute MI, and to compare radiomic signatures among culprit, nonculprit, and stable CAD lesions. By extracting the maximal quantitative information from routine CCTA images, radiomics can provide an insight into fine plaque structure and capture latent traits associated with plaque instability that are typically only explored on the molecular level. We showed textural and geometric features – rather than first-order HU-based metrics – to define the radiomic signature of culprit lesions, and to provide incremental discriminatory value beyond current qualitative and quantitative plaque measures. Textural features characterize plaque composition, expressed by the spatial relationship of voxels on CCTA, while geometric features capture the complex three-dimensional structure of plaques. Certainly, there is evidence from histological studies that the spatial distribution and localization of different plaque components influence plaque vulnerability (17,36). Furthermore, we have previously shown geometric features to associate with echo-attenuated plaque on intravascular ultrasound and thin-cap fibroatheroma on optical coherence tomography (10); potentially reflecting a large lipid pool. Hence, comprehensive CCTA-based plaque analysis integrating conventional parameters with radiomic features may improve our understanding of the morphological factors that act synergistically to increase the risk of ACS.
The “vulnerable patient” concept is well-established (1), and overall coronary atherosclerotic burden quantified by CCTA has shown predictive value for future MI independently of obstructive stenoses or APCs (8,37). Consistent with our prior work (20), the present analysis demonstrated higher volumes and burdens of TP, NCP, and LDNCP in patients with MI compared to patients with stable CAD. Similar results were observed at the lesion level when comparing culprits to nonculprits in MI and to lesions in stable CAD. On radiomic analysis of the entire coronary tree in both cohorts, only the culprit lesion was significantly associated with radiomic features following adjustment for plaque volumes. In single-lesion comparisons, the highest-grade stenosis nonculprit exhibited a distinct radiomic signature compared to the highest-grade stenosis stable CAD lesion. The distinguishing radiomic features were textural and geometric parameters – several of which were also present in culprit lesions – and may reflect vulnerable plaque morphology. Indeed, we showed LDNCP burden to be greater in the highest-grade stenosis nonculprit compared to the highest-grade stable CAD lesion. This is consistent with intracoronary imaging studies demonstrating obstructive nonculprits in MI to more frequently harbor thin-cap fibroatheroma compared to nonobstructive nonculprits (38) or obstructive lesions in stable CAD (39); and suggests that obstructive disease is indirectly associated with subsequent events due to its relationship with plaque burden and morphology. Importantly, recent evidence shows that stenting of nonculprit lesions in MI based on stenosis severity significantly reduces the risk of future MI or cardiac death (40). Overall, our noninvasive imaging findings support the hypothesis that within the vulnerable patient exists one or more vulnerable plaques with distinct morphologies, as evidenced by the radiomic signatures of culprit lesions and highest-grade stenosis nonculprit lesions.
Our study results reflect the morphologies of unstable or disrupted coronary plaques at the time of event, and are hypothesis-generating. It is reasonable to postulate that not-yet-disrupted vulnerable plaques would share similar radiomic features to culprit lesions. Indeed, prior studies have shown that qualitative morphological characteristics do not differ significantly between intact vulnerable plaques and ruptured plaques on CCTA (41,42). Given that established definitions of plaque vulnerability are based on event-causing lesions examined by histology or intracoronary imaging (3,36), the use of CCTA radiomics to phenotype plaque in MI advances the role of noninvasive imaging in the detection of rupture-prone lesions. Future studies should examine plaque radiomic features of stable patients in whom the precursor lesions associated with future ACS have been identified. Moreover, the ability to modify the radiomic signature of the highest-grade stenosis nonculprit lesion in patients with MI through lipid-lowering or anti-inflammatory therapies should be explored. Notably, PROSPECT-ABSORB (Providing Regional Observations to Study Predictors of Events in the Coronary Tree II Combined with a Randomized, Controlled, Intervention Trial) recently showed pre-emptive stenting of nonculprit vulnerable plaques in MI based on intracoronary imaging to be associated with favorable long-term outcomes (43). This paves the way for larger randomized interventional trials, wherein CCTA could be used to help identify candidate vulnerable plaques. Finally, while plaque quantification and radiomic analysis remain investigational, rapid advancements in hardware, software, and artificial intelligence algorithms will facilitate the future implementation of these techniques into clinical practice. In the present study, radiomic extraction followed semi-automated plaque segmentation. The ultimate goal is for fully automated, artificial intelligence-based plaque and radiomic analysis to be embedded into routine CCTA reporting software, providing real-time measurements and flagging vulnerable plaques for clinician review.
Limitations
This study has several limitations. The case-control design may limit the generalizability of our findings to larger, unselected populations. We utilized a small external validation cohort, and our radiomics-based ML model needs further validation in datasets from different centers and CT scanners. There was a predominance of NSTEMI cases, requiring strict adjudication of culprit lesions using invasive angiographic, ECG, and echocardiographic findings. Radiomic analysis was performed on culprit lesions early following MI, and thus the some of the identified radiomic features may reflect post-ACS plaque inflammation. We do not have correlation with intracoronary imaging or histological specimens for the current plaque radiomic signatures, however our prior work has shown radiomic features on CCTA to accurately identify vulnerable plaques as defined by these reference standards in stable patients (10,11).
CONCLUSION
Culprit lesions and highest-grade stenosis nonculprit lesions in MI have distinct radiomic signatures compared with lesions in stable CAD. Within the vulnerable patient, there may exist individual vulnerable plaques which are identifiable using CCTA-based precision phenotyping.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Culprit lesions and highest-grade stenosis nonculprit lesions in MI have distinct radiomic signatures on CCTA compared with lesions in stable CAD. This lends support to the hypothesis that within the vulnerable patient exists individual vulnerable plaques with unique morphologies.
Translational Outlook
CCTA-based radiomic phenotyping of plaque in acute MI advances the role of noninvasive imaging in the detection of the rupture-prone lesions. Future studies should examine whether plaque radiomic features in patients with stable CAD can predict subsequent ACS and are modifiable by conventional or novel therapies.
Funding:
This study was supported in part by grants from the National Heart, Lung, and Blood Institute, USA [1R01HL133616 and 1R01HL148787-01A1]
Abbreviations:
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CP
calcified plaque
- HRP
high-risk plaque
- ICA
invasive coronary angiography
- LDNCP
low-density noncalcified plaque
- MI
myocardial infarction
- ML
machine learning
- NCP
noncalcified plaque
Footnotes
Relationship with industry: Outside of the current work, D.D., S.C., and P.S., received software royalties from Cedars-Sinai Medical Center. D.D. and P.S. hold a patent (US8885905B2 in USA and WO patent WO2011069120A1, Method and System for Plaque Characterization). The remaining authors report no relevant conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arbab-Zadeh A, Fuster V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient. J Am Coll Cardiol 2019;74:1582. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol 2015;65:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanadis C, Antoniou C-K, Tsiachris D, Pietri P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. Journal of the American Heart Association 2017;6:e005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourantas CV, Garcia-Garcia HM, Torii R et al. Vulnerable plaque detection: an unrealistic quest or a feasible objective with a clinical value? Heart 2016;102:581–9. [DOI] [PubMed] [Google Scholar]
- 5.Poon M, Lesser JR, Biga C et al. Current Evidence and Recommendations for Coronary CTA First in Evaluation of Stable Coronary Artery Disease. J Am Coll Cardiol 2020;76:1358–1362. [DOI] [PubMed] [Google Scholar]
- 6.Motoyama S, Ito H, Sarai M et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 7.Chang HJ, Lin FY, Lee SE et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol 2018;71:2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MC, Kwiecinski J, Doris M et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction. Circulation 2020;141:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aerts HJWL. The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review. JAMA Oncology 2016;2:1636–1642. [DOI] [PubMed] [Google Scholar]
- 10.Kolossváry M, Park J, Bang J-I et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. European Heart Journal - Cardiovascular Imaging 2019;20:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolossváry M, Karády J, Kikuchi Y et al. Radiomics versus Visual and Histogram-based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An ex Vivo Study. Radiology 2019;293:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin A, Kolossváry M, Yuvaraj J et al. Myocardial Infarction Associates With a Distinct Pericoronary Adipose Tissue Radiomic Phenotype. J Am Coll Cardiol Img 2020;13:2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS et al. Fourth Universal Definition of Myocardial Infarction (2018). Journal of the American College of Cardiology 2018;72:2231. [DOI] [PubMed] [Google Scholar]
- 14.Wong DT, Soh SY, Ko BS et al. Superior CT coronary angiography image quality at lower radiation exposure with second generation 320-detector row CT in patients with elevated heart rate: a comparison with first generation 320-detector row CT. Cardiovascular diagnosis and therapy 2014;4:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leipsic J, Abbara S, Achenbach S et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 16.Min JK, Shaw LJ, Devereux RB et al. Prognostic Value of Multidetector Coronary Computed Tomographic Angiography for Prediction of All-Cause Mortality. Journal of the American College of Cardiology 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 17.Maurovich-Horvat P, Schlett CL, Alkadhi H et al. The Napkin-Ring Sign Indicates Advanced Atherosclerotic Lesions in Coronary CT Angiography. J AM Coll Cardiol Img 2012;5:1243–1252. [DOI] [PubMed] [Google Scholar]
- 18.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated Three-dimensional Quantification of Noncalcified Coronary Plaque from Coronary CT Angiography: Comparison with Intravascular US. Radiology 2010;257:516–522. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto H, Watanabe S, Kyo E et al. Standardized volumetric plaque quantification and characterization from coronary CT angiography: a head-to-head comparison with invasive intravascular ultrasound. Eur Radiol 2019;29:6129–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dey D, Achenbach S, Schuhbaeck A et al. Comparison of quantitative atherosclerotic plaque burden from coronary CT angiography in patients with first acute coronary syndrome and stable coronary artery disease. J Cardiovasc Comput Tomogr 2014;8:368–74. [DOI] [PubMed] [Google Scholar]
- 21.Cury RC, Abbara S, Achenbach S et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269–81. [DOI] [PubMed] [Google Scholar]
- 22.Kolossvary M RIA: Radiomics Image Analysis Toolbox for Grayscale Images. 2017. https://CRAN.R-project.org/package=RIA.
- 23.Kolossváry M, Karády J, Szilveszter B et al. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign. Circulation: Cardiovascular Imaging 2017;10:e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolossvary M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques. J Thorac Imaging 2018;33:26–34. [DOI] [PubMed] [Google Scholar]
- 25.Kolossváry M, Gerstenblith G, Bluemke DA et al. Contribution of Risk Factors to the Development of Coronary Atherosclerosis as Confirmed via Coronary CT Angiography: A Longitudinal Radiomics-based Study. Radiology 2021;299:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, California, USA: Association for Computing Machinery, 2016:785–794. [Google Scholar]
- 27.Kim J-H. Estimating classification error rate: Repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal 2009;53:3735–3745. [Google Scholar]
- 28.Goeller M, Achenbach S, Cadet S et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol 2018;3:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RC, Nelson GW, Troyer JL et al. Accounting for multiple comparisons in a genome-wide association study (GWAS). BMC Genomics 2010;11:724–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 31.Voros S, Rinehart S, Qian Z et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4:537–48. [DOI] [PubMed] [Google Scholar]
- 32.Puchner SB, Liu T, Mayrhofer T et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motoyama S, Kondo T, Sarai M et al. Multislice Computed Tomographic Characteristics of Coronary Lesions in Acute Coronary Syndromes. Journal of the American College of Cardiology 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa T, Yamamoto H, Horiguchi J et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging 2009;2:153–60. [DOI] [PubMed] [Google Scholar]
- 35.Ferencik M, Schlett CL, Ghoshhajra BB et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. The American journal of cardiology 2012;110:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the Vulnerable Plaque. Journal of the American College of Cardiology 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 37.Lee S-E, Sung JM, Rizvi A et al. Quantification of Coronary Atherosclerosis in the Assessment of Coronary Artery Disease. Circulation: Cardiovascular Imaging 2018;11:e007562. [DOI] [PubMed] [Google Scholar]
- 38.Pinilla-Echeverri N, Mehta SR, Wang J et al. Nonculprit Lesion Plaque Morphology in Patients With ST-Segment-Elevation Myocardial Infarction: Results From the COMPLETE Trial Optical Coherence Tomography Substudys. Circ Cardiovasc Interv 2020;13:e008768. [DOI] [PubMed] [Google Scholar]
- 39.Maejima N, Hibi K, Saka K et al. Morphological features of non-culprit plaques on optical coherence tomography and integrated backscatter intravascular ultrasound in patients with acute coronary syndromes. European Heart Journal - Cardiovascular Imaging 2015;16:190–197. [DOI] [PubMed] [Google Scholar]
- 40.Sheth T, Pinilla-Echeverri N, Moreno R et al. Nonculprit Lesion Severity and Outcome of Revascularization in Patients With STEMI and Multivessel Coronary Disease. Journal of the American College of Cardiology 2020;76:1277. [DOI] [PubMed] [Google Scholar]
- 41.Obaid DR, Calvert PA, Brown A et al. Coronary CT angiography features of ruptured and high-risk atherosclerotic plaques: Correlation with intra-vascular ultrasound. Journal of cardiovascular computed tomography 2017;11:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun EJ, Han JH, Yoo SM, Lee HY, Song IS, White CS. Differences in the CT findings between vulnerable plaque and culprit lesions in acute coronary syndrome. Journal of Cardiovascular Computed Tomography 2018;12:115–117. [DOI] [PubMed] [Google Scholar]
- 43.Stone Gregg W, Maehara A, Ali Ziad A et al. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. Journal of the American College of Cardiology 2020;76:2289–2301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.