Abstract
Introduction
Docosahexaenoic acid (DHA), an omega-3 fatty acid, is important for brain development with possible implications in neurodevelopmental outcomes. In the two-arm, randomised, double-blind, placebo-controlled Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants trial, very preterm infants (<29 weeks’ gestation) were supplemented in high doses of DHA or placebo until they reached 36 weeks’ postmenstrual age. We propose a long-term neurodevelopmental follow-up of these children. This protocol details the follow-up at 5 years of age, which aims to (1) confirm our long-term recruitment capacity and (2) determine the spectrum of neurodevelopmental outcomes at preschool age following neonatal DHA supplementation.
Methods and analysis
This long-term follow-up involves children (n=194) born to mothers (n=170) randomised to DHA (n=85) or placebo (n=85) from the five sites in Quebec when they will be 5 years’ corrected age. The primary outcome measure is related to the long-term recruitment capacity, which we determined as successful if 75% (±10%, 95% CI) of the eligible children consent to the 5-year follow-up study. Interviews with mothers will be conducted to assess various aspects of neurodevelopment at preschool age (executive functions, behavioural problems, global development and health-related quality of life), evaluated with standardised neurodevelopmental questionnaires. In addition, a semistructured interview conducted in a subset of the mothers will be used to determine their acceptability and identify barriers and enablers to their eventual participation to the next phase of the trial. This follow-up study will require approximately 22 months to be completed.
Ethics and dissemination
This study was approved by the CHU de Québec-Université Laval Research Ethics Board (MP-20-2022-5926). Mothers will provide informed consent before participating in this study. Findings will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number
Keywords: neonatal intensive & critical care, neonatology, nutrition & dietetics, paediatrics, developmental neurology & neurodisability, clinical trials
Strengths and limitations of this study.
Building on a well-characterised cohort of breastfed children born very preterm whose mothers were supplemented with high doses of docosahexaenoic acid or placebo, this longitudinal follow-up uses validated tests to assess different aspects of neurodevelopment such as cognition, child behaviour and global development.
The study uses the theoretical domains framework, a well-tested and comprehensive framework, to identify barriers and enablers that may influence the recruitment of the participating children in the next phase of the trial.
The study is conducted in a subset of the Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants cohort, the available sample size might limit study interpretation and the generalisability of the results.
Neurodevelopmental assessment is based on validated standardised questionnaires, which is a good alternative, particularly in the COVID-19 pandemic situation, to formal in-person neuropsychological assessment with performance-based tests.
Introduction
Despite substantial improvements in neonatal care, preterm infants remain at high risk of long-term health complications and adverse neurodevelopmental outcomes.1 Optimising nutrition early in life might represent a clinically applicable strategy to promote brain development and better long-term neurodevelopmental outcomes of very preterm infants.2 Preterm birth is associated with lower levels of docosahexaenoic acid (DHA),3 4 a long-chain polyunsaturated fatty acid implicated in brain development.5 Identifying the brain changes and long-term neurodevelopmental trajectory of preterm infants supplemented with high dose of DHA during the neonatal period is crucial for informing potential nutritional interventions to aim for better outcomes.
DHA is the most abundant omega-3 fatty acid in the brain and is mainly accumulated by the fetal brain during the last trimester of pregnancy.6 As such, during the third trimester, there is an increase in the DHA accretion rate (approximately 60 mg/kg/day).7 Maternal DHA supplementation is an efficient approach to increase DHA concentration in breast milk, which is a safe route to increase DHA levels in the breastfed preterm neonate to match in utero DHA accretion.8 9 Importantly, DHA is implicated in several neuronal functions including neurogenesis, neurotransmission and neuronal protection against inflammation and oxidative stress.5 10–12 In addition, lower DHA levels have been associated with an increased incidence of intraventricular haemorrhage and impaired white matter maturation in preterm infants.13 Hence, optimising nutrition of preterm infants with high dose of DHA (defined as approximately 60 mg/kg/day or 1% DHA of total fatty acids to meet the daily nutritional requirement of very preterm infants14) during the neonatal period might be an efficient strategy to promote brain development and better neurodevelopmental outcomes.
Previous trials assessing the impact of DHA supplementation in preterm infants on neurodevelopmental outcomes have shown inconsistent results.15 16 However, among those studies, very few have evaluated the impact of supplementation with a high dose of DHA (ie, ≥1% of total fatty acids) on the neurodevelopment of preterm infants. In addition, nearly all the trials evaluating the impact of DHA supplementation targeted relatively mature and healthy preterm infants (median gestational age (GA) of 30 weeks for the preterm infants included in the most recent Cochrane review15), who are less vulnerable to DHA deficit and less likely to benefit from a supplementation. Thus, the benefits of supplementation with high doses of DHA on brain development and neurodevelopmental outcomes of very preterm infants born before 29 weeks’ gestation remain to be determined.
We propose a long-term follow-up study of the preterm children involved in the Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants (MOBYDIck) trial to assess the impact of high doses of DHA on the neurodevelopmental trajectory of children born before 29 weeks’ gestation. The following protocol describes the methods for the follow-up at 5 years of age (preschool age) of children whose mothers were enrolled in the MOBYDIck trial. The aims of this study are to (1) confirm our long-term recruitment capacity for future follow-up and (2) determine the spectrum of neurodevelopmental outcomes at preschool age following neonatal DHA supplementation in children born very preterm. Importantly, this follow-up study at preschool age will inform the development of the longer term follow-up planned at school age, which will involve brain imaging and neuropsychological assessment.
Methods and analysis
The MOBYDIck trial: initial study
The MOBYDIck trial is a placebo-controlled randomised clinical trial which aimed to determine whether maternal DHA supplementation during the neonatal period improves bronchopulmonary dysplasia (BPD)-free survival in breastfed infants born before 29 weeks’ GA.17 The study was conducted in 16 Canadian neonatal intensive care units between June 2015 and April 2018. Mothers were eligible for enrolment if they delivered prematurely (between 23 0/7 and 28 6/7 weeks’ GA); if they did not present contraindications for breastfeeding and intended to provide their own breast milk to their infant; and if they were at least 16 years of age. Mothers were excluded if they took more than 250 mg/day of DHA during the 3 months before enrolment, or if their infant had a major congenital or chromosomal abnormality.
Mothers were randomised by site to receive either 1.2 g of DHA daily or a placebo (containing a mix of corn and soy oils) within 72 hours of delivery and up to 36 weeks’ postmenstrual age (PMA). Overall, 232 mothers (273 infants) received DHA supplementation while 229 mothers (255 infants) were in the placebo group. The randomisation was conducted in a 1:1 ratio through computer-generated random lists of treatment allocation. Randomisation lists were generated by an independent statistician. The DHA and placebo capsules had identical taste and look. All participants, researchers, clinicians and pharmacists were blinded to group allocation.
The sample size calculation was determined for the primary outcome BPD-free survival at 36 weeks’ PMA. Neurodevelopment at 18–22 months’ corrected age (CA) was a major secondary outcome. The trial was terminated early, when approximately half of the expected trial participants had been enrolled, due to concern for harm as the DHA treatment did not improve BPD-free survival at 36 weeks’ PMA compared with the placebo (54.9% vs 61.6%; relative risk 0.91, 95% CI 0.80 to 1.04; p=0.18).17
The MOBYDIck trial: neurodevelopmental follow-up at 18–22 months’ CA
Neurodevelopmental outcomes were assessed in 408 preterm children (85.1% of survivors from the original cohort, 213 children in the DHA group and 195 children in the placebo group) at 18–22 months’ CA with the use of neurological examination and the Bayley Scales of Infant and Toddler Development Third Edition (Bayley-III). Secondary outcomes at 18–22 months’ CA included death and/or significant neurodevelopmental impairment, and the Bayley-III scores in categories.18 All participants, researchers and clinicians responsible for the Bayley-III assessment were blinded to group allocation.
Participants and recruitment
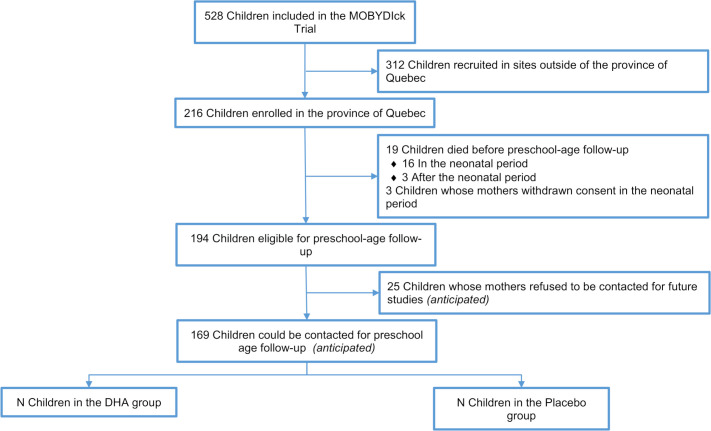
Children of mothers enrolled in the MOBYDIck trial in any of the five sites from the province of Quebec (CHU de Québec-Université Laval, CHU de Sherbrooke, CHU Sainte-Justine, Jewish General Hospital and McGill University Health Centre) will be eligible for this study when they are 5 years’ CA (range from 57 to 66 months’ CA). More specifically, children who survived and had not withdrawn from the trial, from families who have initially indicated their willingness to be contacted for future research projects will be eligible. This subsample was selected as these patients will be the ones eligible for the planned follow-up at 8 years of age. Among the 216 children born to mothers enrolled in the five Quebec sites, 194 will be eligible for follow-up study at 5 years of age once deaths (n=19) and withdrawals (n=3) are excluded. Based on the information provided on the original MOBYDIck consent form from one of the sites, we anticipate that a total of 169 children could be contacted (as their mothers will have indicated their agreement to be contacted for future studies (anticipated exclusion n=25)) (figure 1). Participants and researchers will remain blinded to treatment group allocation.
Figure 1.
MOBYDIckPS participant flow chart. DHA, docosahexaenoic acid; MOBYDIck, Maternal Omega-3 Supplementation to Reduce Bronchopulmonary Dysplasia in Very Preterm Infants.
The mothers of eligible children will be invited by email to participate in this follow-up study when their child will be close to 5 years’ CA (ie, ≥57 months’ CA). Mothers of eligible children will be provided with all relevant information about the study and will be able to provide their informed consent via a secure online platform. A member of the research team will be available to answer their questions and will confirm understanding of the study procedures. Mothers who accept to participate will be contacted to set up a time for an interview, which will be conducted when their child reaches 5 years’ CA, with an acceptable range for interviews to be conducted between 57 and 66 months’ CA. In case an interview cannot be performed before 66 months’ CA, interviews will still be conducted with age-appropriate questionnaires.
On September 1st, 2021, eighty-nine children will be 57 months’ CA or above (n=21 will be ≥66 months’ CA), and therefore eligible for the study. In April 2023, all children will be >57 months’ CA. To complete this follow-up study from start (August 2021) to the assessment of the last participating child will require approximately 22 months (last assessment expected in July 2023).
Outcomes and measures
Primary outcome
The primary outcome is related to the long-term recruitment capacity for a future follow-up study planned at school age and will be assessed based on the proportion of eligible children recruited for preschool-age assessment. We established that recruitment is considered successful if 75% (±10%, 95% CI) of the eligible children consent to the preschool follow-up study. As detailed in table 1, a minimal sample size of 80 participants, out of the 169 anticipated eligible children (see figure 1), is needed to estimate this recruitment capacity.19
Table 1.
Sample size to establish a recruitment capacity of 75%
| CI | Sample size | Proportion | Lower limit | Upper limit | |
| 75% of recruitment capacity | 0.95 | 80 | 0.75 | 0.641 | 0.840 |
Secondary outcomes
Acceptability of the next phase of the study
The secondary outcomes are related to the acceptability of a future trial planned at 8 years of age, which will include for the child to complete a brain MRI and neuropsychological assessment. Data will include reasons for not being interested in participating in the 8-year study, views of parents on this future follow-up and identification of potential barriers and facilitators that may influence their participation. To assess parents’ acceptability of the proposed study we will apply the theoretical domains framework (TDF), a systematic and comprehensive approach to address barriers in research implementation.20 21 The TDF is an approach used to develop semistructured interviews with questions based on 14 domains that describe factors that may act as barriers or enablers that may influence participation to a trial (table 2). Data from these interviews will be analysed using NVivo (QSR International) with standard recommendation for TDF-based qualitative study analysis.22
Table 2.
Theoretical domains framework domains, definitions and associated questions for semistructured interviews
| TDF domain | Definitions (Cane et al)20 | Semistructured interview question |
| Knowledge |
An awareness of the existence of something. |
Do you know what an MRI is? Have you or somebody you know ever had a brain MRI? |
| What information would you need to feel comfortable with your child having an MRI performed as part of a research trial? | ||
| Skills | An ability or proficiency acquired through practice. | Before considering if ‘name of the child’ will participate or not in this study, who would you talk to? |
| Social/professional role and identity | A coherent set of behaviours and displayed personal qualities of an individual in a social or work setting. | How would enrolling ‘name of the child’ into a study involving brain MRI would fit with how you see yourself as a parent and what is important to you? Prompt: Challenge your values? Any moral or ethical issues? |
| Beliefs about capabilities | Acceptance of the truth, reality or validity about an ability, talent or facility that a person can put to constructive use. | On a scale of 0–10, how confident are you that you could decide to enrol ‘name of the child’ in a study involving brain MRI at school age? And why? |
| Optimism | The confidence that things will happen for the best or that desired goals will be attained. | Do you expect that participating in this study will result in more good things than bad things for yourself and ‘name of the child’? |
| Beliefs about consequences | Acceptance of the truth, reality or validity about outcomes of a behaviour in a given situation. | What are some of the potential negative aspects or problems that you see in participating in clinical trial involving an MRI of the brain? |
| Reinforcement | Increasing the probability of a response by arranging a dependent relationship, or contingency, between the response and a given stimulus. | Is there any personal incentive, like a financial compensation, that would have an impact in the participation of your child in this study? Are there any other incentives that could facilitate your participation in the study? |
| Intentions | Conscious decision to perform a behaviour or a resolve to act in a certain way. | Based on what you’ve learnt so far on MRI for the brain, would you agree for ‘name of the child’ to participate in the next phase of the study and have a brain MRI and developmental assessment at around 8 years of age? |
| Goals | Mental representations of outcomes or end states that an individual wants to achieve. | What would motivate you to involve ‘name of the child’ in this study? Prompts: Obtain more information on ‘name of the child’ brain development, contribute to research, health benefits for ‘name of the child’. |
| Memory, attention and decision processes | Ability to retain information, focus selectively on aspects of the environment and choose between two or more alternatives. | If you heard about a follow-up study available for ‘name of the child’, involving brain MRI and developmental assessment, how would you like to be contacted for more details? |
| Environmental context and resources |
Any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence, an adaptive behaviour. |
What would be the best format for you to receive more information about brain MRI? What resources do you need to be made available for your child to participate in this research study? |
| The proposed study at age 8 years will likely involve two visits (MRI: study visit 1–2 hours, developmental assessment visit: 2 hours), would this frequency and length of appointment influence your decision to participate? | ||
| Social influences | Interpersonal processes that can cause individuals to change their thoughts, feelings or behaviours. | From whom would you like to receive information about MRI? When you consider if ‘name of the child’ will participate or not in this study, whose opinion is important for you? Who would influence your decision the most? |
| Emotions | Complex reaction pattern, involving experiential, behavioural and physiological elements, by which the individual attempts to deal with a personally significant matter or event. | When you imagine ‘name of the child’ participating in this study, what emotions come to your mind? How would you feel about ‘name of the child’ having a brain MRI when he/she will be 8 years of age? Prompts: Satisfaction, worry, happy, sad, nervous, confident. |
TDF, theoretical domains framework.
Secondary neurodevelopmental outcomes
The secondary neurodevelopmental outcomes include various measures to assess the spectrum of the children neurodevelopment at 5 years of age (table 3):
Table 3.
Neurodevelopmental questionnaires at preschool age
| Dimension | Standardised questionnaire | Description | Time (min) |
| General health/quality of life | Pediatric Quality of Life Inventory (PedsQL) |
|
<5 |
| Behavioural problems | Strengths and Difficulties Questionnaire (SDQ) |
|
5 |
| Executive functioning | Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P) |
|
10–15 |
| Global development | Ages and Stages Questionnaire (ASQ) |
|
10 |
Child health-related quality of life assessed by the Pediatric Quality of Life Inventory (PedsQL). The PedsQL is a brief and standardised questionnaire designed to measure health-related quality of life in children.23
Behavioural problems assessed by the Strengths and Difficulties Questionnaire (SDQ). The SDQ is a short behavioural screening questionnaire.24
Child’s executive functioning assessed by the Behavior Rating Inventory of Executive Function-Preschool, a questionnaire designed to be completed by caregiver to assess executive functions in daily environments of children.25
Global development assessed by the Ages and Stages Questionnaire, an age-specific developmental screening.26 We will use the 60-month questionnaire (applicable to children between 57 and 66 months) or the 54-month questionnaire (applicable from 51 through 56 months and 30 days), as appropriate.
Exploratory outcome
Exploratory outcome includes documenting the COVID-19 pandemic exposure and its impact on the home environment using an 8-item questionnaire. This questionnaire is designed to evaluate children and parents’ exposure to COVID-19, its impact on the child’s environment, the effects of quarantine and the financial implications related to COVID-19 pandemic for the family.
Data collection
In addition to the semistructured interview and the various questionnaires, data will be collected on the medical status of the participating children and the familial socioeconomic status. Of note, the semistructured interview to identify acceptability, potential barriers and facilitators to an MRI study will be audio recorded once consent is obtained. The recordings will be transcribed verbatim and verified by the interviewer. All identifying information will be removed from the interview transcripts.
Questionnaires will be completed by our research team, trained in conducting TDF interviews, with the mothers over the phone or via a videoconferencing call and is expected to last approximately 60 min.
Sample size calculation
We established that recruitment is considered successful if 75% (±10%, 95% CI) of the eligible children participate to the 5-year follow-up study. We anticipated that if 75% of the 169 eligible children are included in the 5-year follow-up study and if 90% (95% CI) of these children complete the assessment (ie, interview completed with the mother), we will obtain data on a total of 96–108 participants. This proposed sample size is largely sufficient for an MRI study at 8 years of age.
Statistical analysis
For the primary outcome, recruitment capacity will be calculated based on the proportion of eligible children enrolled in the study. A minimum of 80 participants is needed to establish a successful recruitment capacity (see details in table 1). For the secondary outcomes related to the acceptability of the 8-year study, qualitative analyses, aligned with TDF-based qualitative study analysis,22 will be done for the semistructured interview section to determine the parents’ acceptability and to identify potential barriers and facilitators to their participation. Data from this section of the interviews will be analysed using NVivo (QSR International). We will conduct TDF-based semistructured interviews until achievement of data saturation. In accordance with the principle of data saturation established for qualitative studies, there is evidence suggesting that data saturation is achieved when no new concepts emerge from three consecutive interviews, after an established minimum initial sample size of 10.27 If new concepts are raised, interviews will continue to be conducted until three consecutive interviews do not raise new themes. Once data saturation is achieved, this section of the interviews (ie, school-age study acceptability semistructured interviews) will be removed from the questionnaires with the remaining families.
For neurodevelopmental outcomes and baseline characteristics, descriptive statistics will be presented as n (%) for categorical variables and as mean (SD) for continuous variables. The treatment effect will be measured using generalised estimating equation models adjusted for clustering of children due to multiple birth.
Data management
The Collaboration pour l’Efficacité en Diagnostic (CRED) informatics platform, certified ISO 27001, will provide the Programme Informatique En Recherche Collaborative (PIERCE) software, a secure web-based electronic data capturing platform, to develop a database and collect data from the interviews conducted with mothers. The consent form will also be available online using the same secure web platform (PIERCE). The CRED informatics platform is part of the Research Centre of the Centre Hospitalier Universitaire de Sherbrooke and works to promote secure and web-based applications to facilitate collaborative research. Only researchers associated with the study will have access to the individual participant data.
Participants who agreed to participate will have their contact information kept in a secure location at the research coordination centre (CHU de Québec-Université Laval). All records will be retained for 25 years after the study closure.
Ethics and dissemination
This follow-up study is approved by the CHU de Québec-Université Laval Research Ethics Board (MP-20-2022-5926). We do not anticipate any risks or direct benefits for eligible children who will be enrolled in this follow-up
The results of this follow-up study will be presented at relevant national and international meetings and published in peer-reviewed journals.
Patient and public involvement
The interview guides for this follow-up study were developed in collaboration with the parent of a very preterm infant. In addition, an important objective of this proposed follow-up study is to address parents’ views and concerns and determine their willingness to participate in a future follow-up study at school age. Hence, this study involves the patient (and their parent) in the very first phases of developing the next steps of the trial.
Discussion
This follow-up study in a subset of participants of the MOBYDIck trial will help determine the impact of maternal DHA supplementation during the neonatal period on brain development and neurodevelopmental outcomes of breastfed very preterm infants. As such, this follow-up at preschool age is part of a longer term longitudinal cohort study, which will include brain imaging with MRI and neurodevelopmental assessment at 8 years of age. This proposed longitudinal follow-up study is unique and will contribute to better understand the role of DHA supplementation during the neonatal period on brain development and the neurodevelopmental trajectory of high-risk preterm infants.
This follow-up study at preschool age will inform the development of the longer term follow-up planned at school age by establishing our long-term recruitment capacity, quantifying the acceptability and addressing parents’ views and concerns early in the process. This study will also contribute to better identify the characteristics of participants lost to follow-up. The success of a longitudinal cohort study relies on several factors including recruitment capability, acceptability of the proposed study procedures, development of data collection procedures and identification of outcome measures. The results of this study will address these key factors and will improve the design of the next steps of the trial.
In addition, our follow-up at preschool age will contribute to determine the neurodevelopmental trajectory after DHA supplementation in preterm infants. As such, we will benefit from a longitudinal neurodevelopmental assessment of the children included in the MOBYDIck trial with a first formal neurodevelopmental assessment already completed at 18–22 months’ CA, followed by an assessment at 5 years (preschool) and a complete evaluation, including brain MRI and neuropsychological assessment planned at 8 years (school age). This longitudinal follow-up will be important to better identify the impact of maternal DHA on brain development and long-term outcomes of this high-risk population. Importantly, neurodevelopmental assessment at preschool age and school age is particularly relevant to measure specific domains (eg, cognition, executive functioning, behavioural difficulties, etc) which might interfere with school success.
Furthermore, considering the association between DHA supplementation and higher rates of BPD detected during the initial part of the MOBYDIck trial, this follow-up is important to identify potential long-term benefit on neurodevelopment. Indeed, the adverse effect of DHA on BPD could be counterbalanced by a significant positive effect on neurodevelopment, which needs to be further studied.
Our study has some limitations. First, due to the early trial termination, the sample was smaller than planned. In addition, the sample size of the MOBYDIck trial was calculated to detect a difference in BPD-free survival. Hence, neurodevelopmental results should be interpreted cautiously. Still, most of the studies assessing the impact of DHA on the neurodevelopment of preterm infants report on smaller cohorts than ours. Second, our assessment at preschool age relies on standardised questionnaires completed through interviews with the mothers. Although these neurodevelopmental questionnaires are reliable and have been previously validated, they should be considered as screening tools and are limited in comparison to a formal neuropsychological assessment with performance-based tests. In addition, some of these neurodevelopmental questionnaires are typically self-completed by parents. While including these questionnaires in the interviews ensured the questions were fully answered and allowed the parent to ask questions as needed, this approach might have influenced the results. Importantly, in case the questionnaires raised concerns regarding the child’s neurodevelopment and health, the families were provided with adequate resources.
In conclusion, with the improvement in the survival of very preterm infants over the last few decades, it is now a priority to identify clinically applicable strategies to improve their longer term outcomes.1 Although preclinical studies suggest a positive impact of DHA on brain development,10–12 the impact of high-dose DHA supplementation on brain development and neurodevelopmental outcome of very preterm infants remains to be determined. The longitudinal follow-up of the MOBYDIck randomised controlled trial offers an ideal and unique opportunity to address this question within a cohort of well-characterised very preterm children.
Supplementary Material
Acknowledgments
We would like to thank the participating children and their families for their well-appreciated contribution to the MOBYDIck trial.
Footnotes
Contributors: MG and EP conceptualised and designed the follow-up study, and drafted and revised the manuscript. C-AR and LT contributed to data acquisition and analysis, and drafted and revised the manuscript. GC, CM-G, FO, JB, EM, AM and IMo conceptualised and designed the follow-up study, and revised the manuscript. IMa conceptualised and designed the initial and follow-up study, provided study supervision, obtained funding, and drafted and revised the manuscript.
Funding: The MOBYDIck trial was funded by the Canadian Institutes of Health Research (CIHR, grant MOP-136964).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Cheong JLY, Anderson PJ, Burnett AC, et al. Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics 2017;139. 10.1542/peds.2016-4086 [DOI] [PubMed] [Google Scholar]
- 2.Ottolini KM, Andescavage N, Keller S, et al. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediatr Res 2020;87:194–201. 10.1038/s41390-019-0508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foreman-van Drongelen MMHP, van Houwelingen AC, Kester ADM, et al. Long-Chain polyunsaturated fatty acids in preterm infants: status at birth and its influence on postnatal levels. J Pediatr 1995;126:611–8. 10.1016/S0022-3476(95)70363-2 [DOI] [PubMed] [Google Scholar]
- 4.Lapillonne A, Groh-Wargo S, Lozano Gonzalez CH, Gonzalez CHL, et al. Lipid needs of preterm infants: updated recommendations. J Pediatr 2013;162:S37–47. 10.1016/j.jpeds.2012.11.052 [DOI] [PubMed] [Google Scholar]
- 5.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr 2007;137:855–9. 10.1093/jn/137.4.855 [DOI] [PubMed] [Google Scholar]
- 6.Smith SL, Rouse CA. Docosahexaenoic acid and the preterm infant. Matern Health Neonatol Perinatol 2017;3:22. 10.1186/s40748-017-0061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadders-Algra M, Bouwstra H, van Goor SA, et al. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med 2007;35:S28–34. 10.1515/JPM.2007.034 [DOI] [PubMed] [Google Scholar]
- 8.Collins CT, Sullivan TR, McPhee AJ, et al. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids 2015;99:1–6. 10.1016/j.plefa.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Marc I, Plourde M, Lucas M, et al. Early docosahexaenoic acid supplementation of mothers during lactation leads to high plasma concentrations in very preterm infants. J Nutr 2011;141:231–6. 10.3945/jn.110.125880 [DOI] [PubMed] [Google Scholar]
- 10.Sidhu VK, Huang BX, Kim H-Y. Effects of Docosahexaenoic Acid on Mouse Brain Synaptic Plasma Membrane Proteome Analyzed by Mass Spectrometry and 16 O/ 18 O Labeling. J Proteome Res 2011;10:5472–80. 10.1021/pr2007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Layé S, Nadjar A, Joffre C, et al. Anti-Inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharmacol Rev 2018;70:12–38. 10.1124/pr.117.014092 [DOI] [PubMed] [Google Scholar]
- 12.Harel T, Quek DQY, Wong BH, et al. Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics 2018;19:227–35. 10.1007/s10048-018-0556-6 [DOI] [PubMed] [Google Scholar]
- 13.Tam EWY, Chau V, Barkovich AJ, et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr Res 2016;79:723–30. 10.1038/pr.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould JF, Roberts RM, Makrides M. The influence of omega-3 long-chain polyunsaturated fatty acid, docosahexaenoic acid, on child behavioral functioning: a review of randomized controlled trials of DHA supplementation in pregnancy, the neonatal period and infancy. Nutrients 2021;13:415. 10.3390/nu13020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon K, Rao SC, Schulzke SM, et al. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Reviews 2016;2017:CD000375. 10.1002/14651858.CD000375.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newberry SJ, Chung M, Booth M, et al. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evid Rep Technol Assess 2016;224:1–826. 10.23970/AHRQEPCERTA224 [DOI] [PubMed] [Google Scholar]
- 17.Marc I, Piedboeuf B, Lacaze-Masmonteil T, et al. Effect of maternal docosahexaenoic acid supplementation on bronchopulmonary Dysplasia–Free survival in breastfed preterm infants. JAMA 2020;324:157–67. 10.1001/jama.2020.8896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillot M, Synnes A, Pronovost E, et al. Maternal high-dose DHA supplementation and neurodevelopment at 18-22 months of preterm children. (In Press). [DOI] [PubMed]
- 19.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–12. 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- 20.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implementation Sci 2012;7:37. 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michie S, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Quality and Safety in Health Care 2005;14:26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins L, Francis J, Islam R, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implementation Sci 2017;12:77. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. 10.1097/00005650-199902000-00003 [DOI] [PubMed] [Google Scholar]
- 24.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol & Psychiat 1997;38:581–6. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 25.Gioia GA, Espy KA, Isquith PK. The behavior rating inventory of executive Function-Preschool version (BRIEF-P). Odessam FK: Psychological Assessment Resources, 2003. [Google Scholar]
- 26.Squires J, Bricker D. Ages and stages questionnaire, 3rd Edn. (ASQ-3TM). Baltimore: Brookes Publishing, 2009. [Google Scholar]
- 27.Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229–45. 10.1080/08870440903194015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.