Fig. 4.
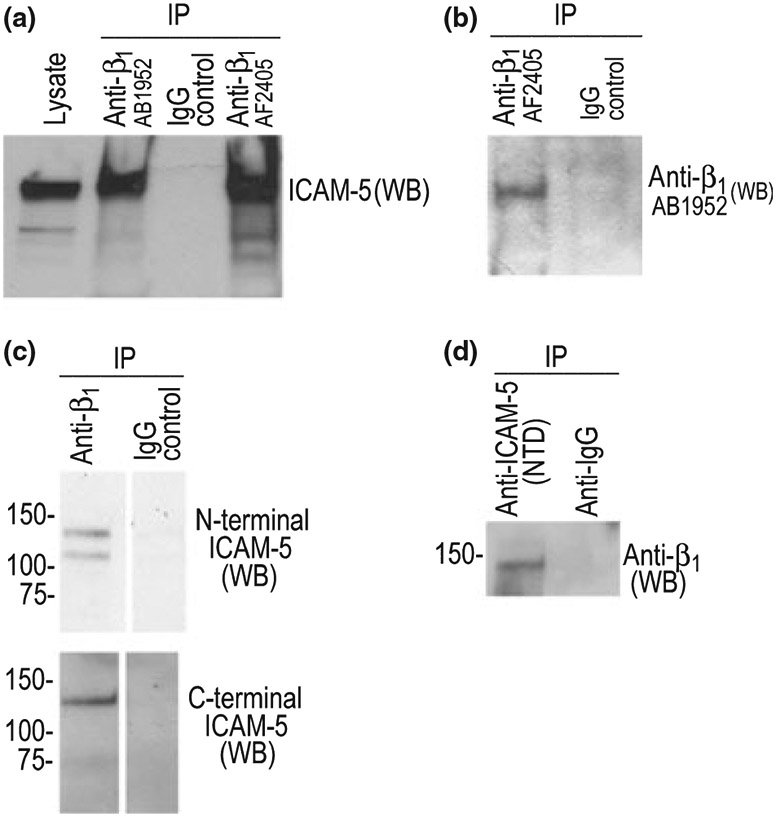
The ectodomain of ICAM-5 and β1 co-immunoprecipitate B35 cells, which had previously been determined to express β1 but not ICAM-5, were treated with recombinant ICAM-5. B35 cells were treated with 1 μg/mL ICAM-5 for 30 min. prior to lysate preparation. Anti-integrin or control (IgG) antibodies (Millipore) were then used to pull out β1 and potential interacting proteins from the ICAM-5 treated B35 cells. Western blot was later performed for ICAM-5 (a) or, as an additional control, β1 (b). ICAM-5 treatment of cells and immunoprecipitation was performed as previously described for MMP-1 (Conant et al. 2004). To determine whether ICAM-5 might interact with β1 in vivo, we also performed studies using lysate from MA treated (6 h) murine hippocampus. As shown in panel c, anti-β1 pulled down both the full length (detected by both N- and C-terminal ICAM-5 antibodies) and ectodomain (detected only by the N-terminal antibody) of ICAM-5. As shown (d), an antibody to the N-terminal domain of ICAM-5 can pull down β1.
