1H and 13C NMR data of 1–2 and 5 in CDCl3a.
| Position | 1 | 2 | 5 | |||
|---|---|---|---|---|---|---|
| δ H | δ C | δ H | δ C | δ H | δ C | |
| 1 | 4.50 d (6.5) | 65.2 | 4.52 d (6.5) | 65.4 | 170.9 | |
| 2 | 5.56 dt (15.0, 6.5) | 124.0 | 5.58 dt (15.0, 6.5) | 124.1 | 5.83 d (15.5) | 124.1 |
| 3 | 5.75 dt (15.0, 7.0) | 136.2 | 5.78 dt (15.0, 6.5) | 136.5 | 6.83 dt (15.5, 7.0) | 146.5 |
| 4 | 2.06 m | 32.0 | 2.06 mb | 32.1 | 2.22 dt (7.0, 8.0) | 31.0 |
| 5 | 1.40 m | 28.4 | 1.42 dt (7.0, 3.5) | 28.8 | 1.33 mb | 24.8 |
| 6 | 1.40 m | 28.4 | 1.42 dt (7.0, 3.5) | 28.5 | 1.58 mb | 27.9 |
| 7 | 2.06 m | 32.0 | 2.06 mb | 32.2 | 5.43 dt (11.0, 7.5) | 130.6 |
| 8 | 5.75 dt (15.0, 7.0) | 136.2 | 5.71 dt (15.0, 6.5) | 133.2 | 6.04 t (11.0) | 128.5 |
| 9 | 5.56 dt (15.0, 6.5) | 124.0 | 5.65 dt (15.0, 6.0) | 129.3 | 6.51 dd (15.0, 11.0) | 125.0 |
| 10 | 4.50 d (6.5) | 65.2 | 4.10 d (6.0) | 63.9 | 5.65 dd (15.0, 7.0) | 136.2 |
| 11 | 4.10 q (7.0) | 71.9 | ||||
| 12 | 1.48 m, 1.54 mb | 36.9 | ||||
| 13 | 2.26 m | 26.6 | ||||
| 14 | 1.33 mb | 31.5 | ||||
| 15 | 1.33 mb | 22.3 | ||||
| 16 | 0.93 t (7.0) | 12.9 | ||||
| 1-MeC̲O | 170.9 | 171.0 | ||||
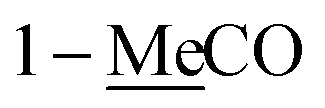
|
2.06 s | 21.0 | 2.08 s | 21.2 | ||
| 10-MeC̲O | 170.9 | |||||
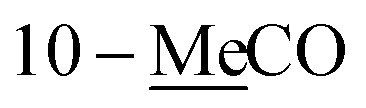
|
2.06 s | 21.0 | ||||
Signal multiplicity and coupling constants (Hz) are in parentheses. The assignments were based on HSQC, HMBC, and TOCSY/1H–1H COSY experiments; 1H and 13C NMR were measured using NMR spectrometers operating at 700 MHz (1H) and 175 MHz (13C) for compounds 1 and 2, and 600 MHz (1H) and 150 MHz (13C) for compound 5.
Overlapped.
