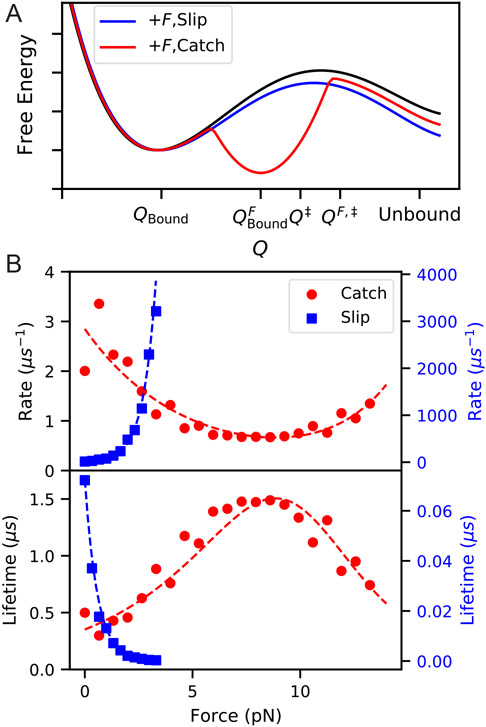
Figure 9:
(A) Schematic of a free energy landscape for an unbinding reaction (black), which with applied force F behaves as either a slip bond (blue) or catch bond (red). For models of a catch bond projected into a 1D reaction coordinate Q, we imagine that application of force creates a new stable minimum which in turn has a higher barrier for transition to the same unbound state. (B) Illustration of catch and slip bond kinetics computed by preliminary MD simulations using infrequent metadynamics52 on 2D potentials in pesmd.53 The slip bond potential is a simple double well aligned with the x-axis, with force applied along the x-axis. The catch-bond potential is a modification of the titled double-well potential from Ref. 10, with a third local minimum added in such that the third state becomes the most stable at intermediate force. Dashed lines show fits to Bell’s law for slip-bonds and the two-pathway model for catch bonds.54