Figure 2. Resistance to BTK Inhibitors Conferred by BTK Mutations Outside the C481 Residue.
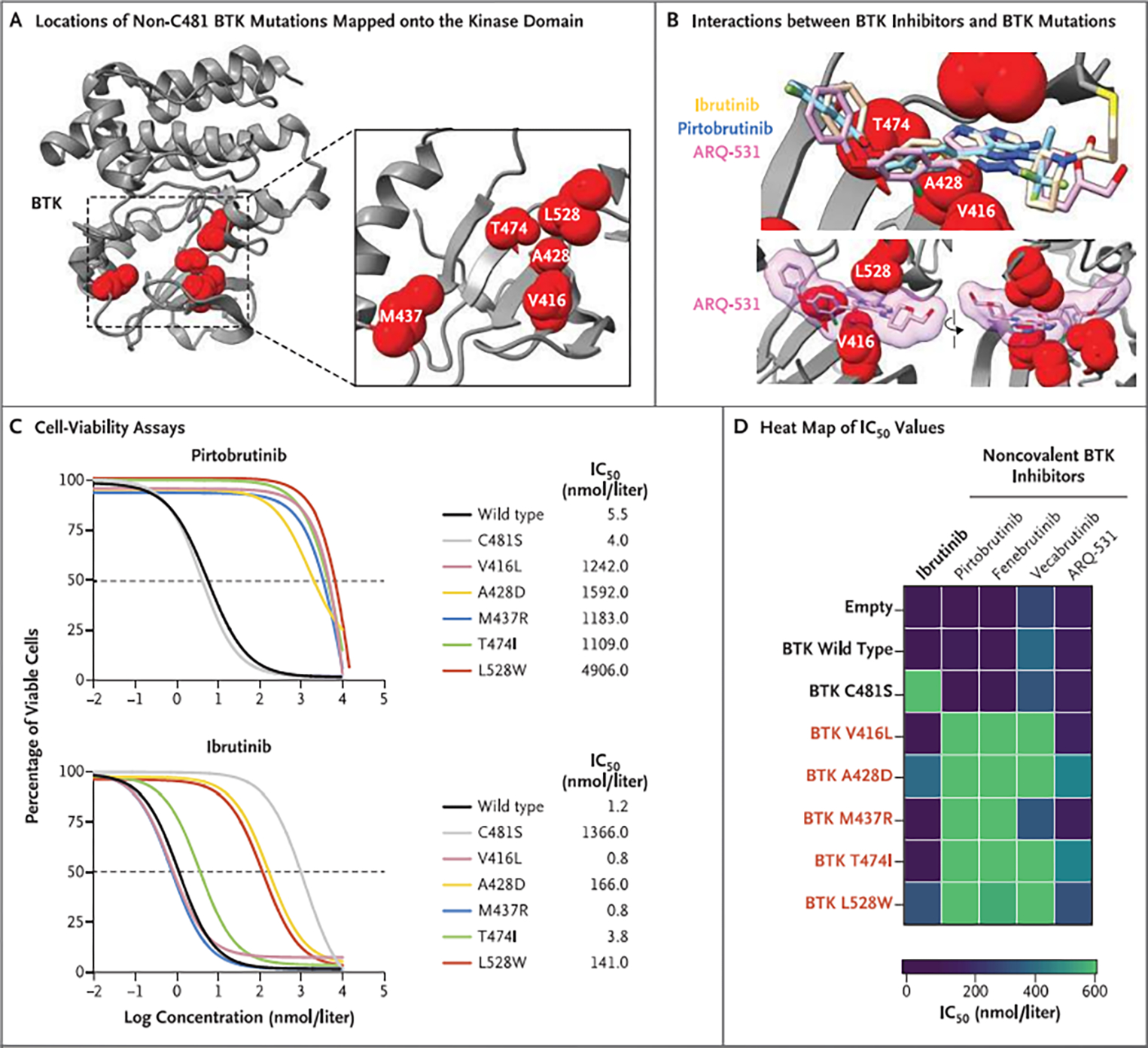
Panel A shows the locations of non-C481 BTK mutations (V416L, A428D, M437R, T474I, and L528W) identified in patients with pirtobrutinib resistance mapped onto the crystal structure of the BTK kinase domain (gray ribbon). Mutated amino acids are shown as red spheres. Panel B shows the chemical structures of ibrutinib, pirtobrutinib, and ARQ-531 overlaid onto the crystal structure of the BTK kinase domain to illustrate the interactions between noncovalent BTK inhibitors and the non-C481 BTK mutations identified in specimens from patients with pirtobrutinib resistance. Panel C shows the results of experiments in which TMD8 cells transduced with mutant BTK were treated with pirtobrutinib or ibrutinib for 72 hours and the half-maximal inhibitory concentrations (IC50) determined with the use of cell-viability assays. Panel D shows a heat map of IC50 values at 72 hours after treatment of TMD8 cells transduced with mutant BTK with ibrutinib or a panel of noncovalent BTK inhibitors (pirtobrutinib, fenebrutinib, vecabrutinib, or ARQ-531). These data show that BTK C481S is resistant to ibrutinib but sensitive to noncovalent BTK inhibitors, whereas cells expressing BTK mutants V416L, A428D, M437R, T474I. and L528W are less sensitive to noncovalent BTK inhibitors. The data in Panels C and D are from three independent replicates and have been normalized to values obtained with a vehicle control (dimethylsulfoxide).
