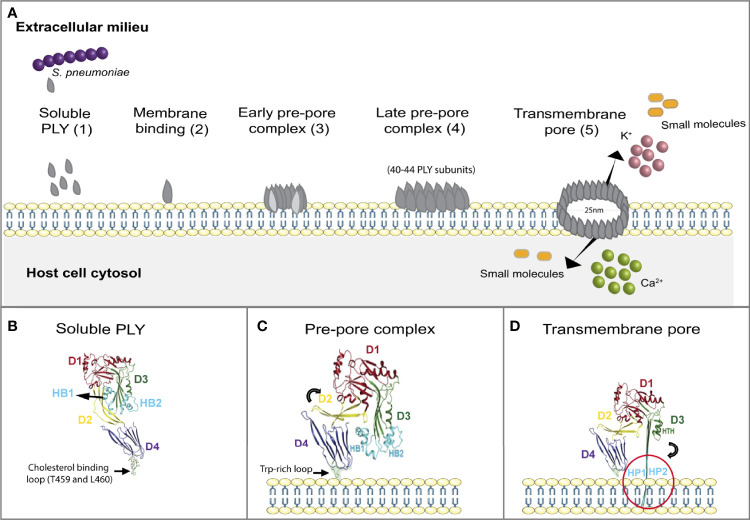
Figure 2.
Mechanisms of PLY-mediated host plasma membrane permeabilization and conformational changes associated to pore formation. (A) PLY pore-formation is a multi-step process. PLY is released by S. pneumoniae as a water-soluble monomer (1) which specifically bind to cholesterol residues on the host cell plasma membrane (2). PLY monomers oligomerize by interacting with each other to form the early pre pore complex (3), which protrudes into the membrane surface establishing the late pre-pore (4). Finally, PLY inserts hairpins HP1 and HP2 across the membrane forming an open transmembrane channel, which allows the uncontrolled influx and efflux of ions and small molecules (5). (B) Soluble PLY. The 3D crystal structure of PLY monomer as it is released from S. pneumoniae is shown. The 4 major domains, from D1 to D4, as well as Helix Bundles (HB) 1 and 2 and cholesterol binding loop are indicated. The arrow indicates residues T459 and L460 in the D4 Trp-rich loop which are essential for cholesterol recognition. (C) PLY in pre-pore complex. Structure of PLY upon cholesterol binding via the conserved D4 Trp-rich loop. Interaction with cholesterol induces a 90° rotation of D2 (in yellow, indicated by the curved arrow) bringing D1and D3 towards the host plasma membrane. HB1 and HB2 are positioned just above the host membrane. This structural organization is maintained in the pre-pore stage. (D) PLY in the transmembrane pore. The 3D structure of PLY when inserted in the host cell plasma membrane is depicted. HB1 and HB2 refold into 85 Å β -hairpins HP1and HP2 (shown by the curved arrow), which insert (red circle) and cross the hydrophobic membrane to form an open transmembrane pore. Adapted from (42).