FIGURE 1.
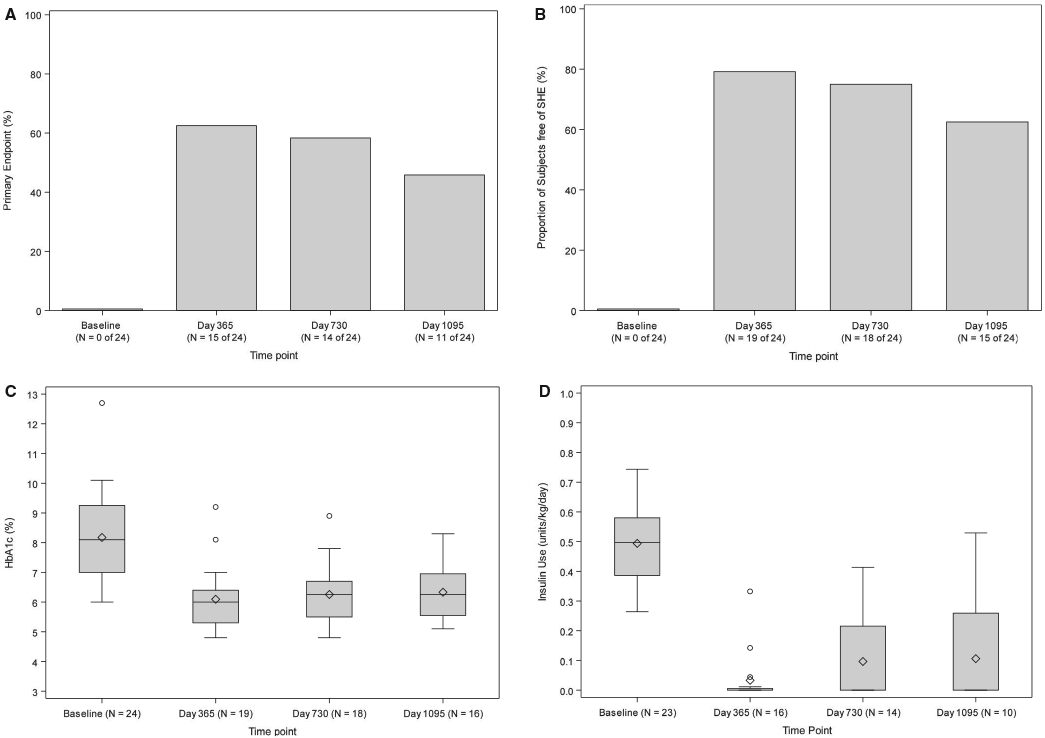
A, primary endpoint (%). An exact 1-sided test for a proportion of ≤.27 vs >0.27 was performed at day 365, day 730, and day 1095. The P values were P = .0003 at day 365, P = .0012 at day 730, and P = .0369 at day 1095. The threshold for statistical significance based on the Benjamini-Hochberg (BH) false discovery rate (FDR) method was 0.0167. B, Free of severe hypoglycemic event (SHE) (%). An exact 1-sided test for proportion ≤0.5 vs >0.5 was performed at day 365, day 730, and day 1095. The P values were P = .0033 at day 365, P = .0113 at day 730, and P = .1537 at day 1095. The threshold for statistical significance based on the BH FDR method was 0.0167. C, HbA1c (%). The Wilcoxon signed rank test for paired outcomes was used to compare the HbA1c levels (%) between baseline and day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The BH method was used to adjust the level of significance in order to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P < .0001 for day 365, P = .0016 for day 730, and P = .0002 for day 1095. D, Insulin use (U/kg/d). The Wilcoxon signed rank test for paired outcomes was used to compare insulin use (U/kg/d) at day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance in order to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P < .0001 for day 365, P = .0001 for day 730, and P = .0020 for day 1095
