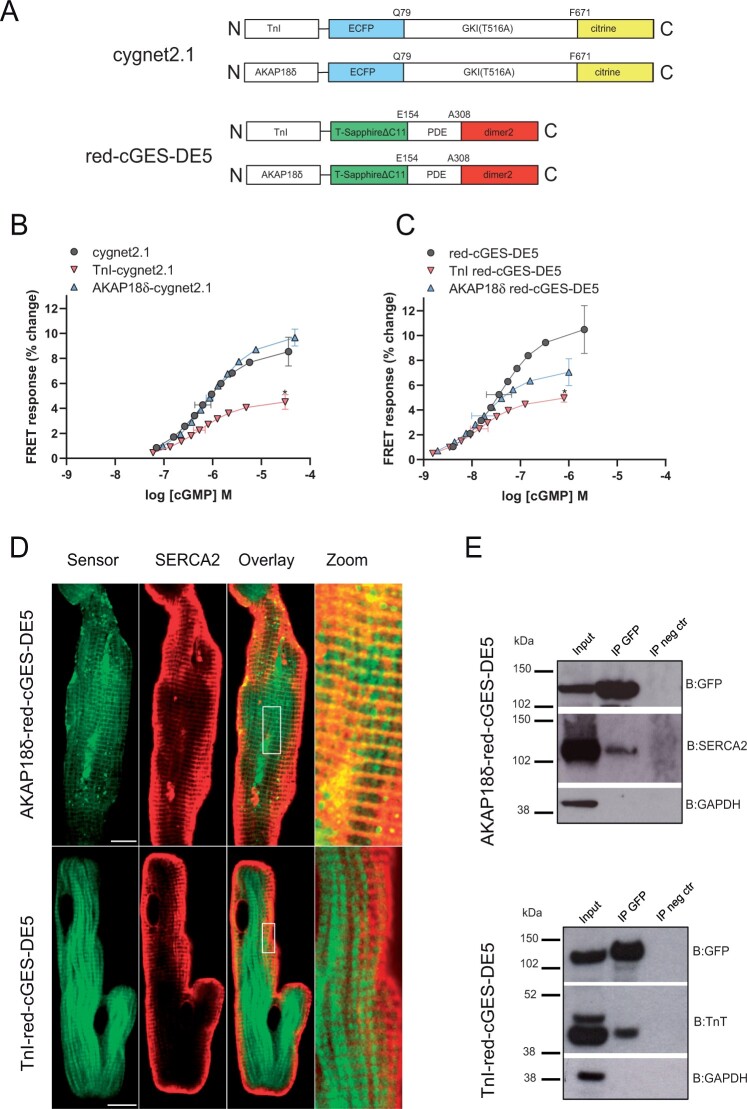
Figure 1.
Characterization of TnI- and AKAP18δ-targeted cGMP biosensors. (A) Schematic domain structures of cGMP FRET-based biosensors targeted to TnI and AKAP18δ. Cygnet2.1 includes a cGMP binding domain from a truncated version of protein kinase GI (GKI) with a T516A mutation sandwiched between ECFP and citrine. Red-cGES-DE5 includes the GAF-A domain from PDE5 sandwiched between T-Sapphire and dimer2. TnI or AKAP18δ were fused to an alpha helix on the N-terminus of the biosensors. (B, C) HEK293T cells transfected with the indicated Cygnet 2.1 (B) or red-cGES-DE5 (C) biosensors were homogenized and incubated with increasing concentrations of cGMP. ECFP or T-Sapphire were excited at 430nm or 405nm, respectively, and the fluorescent emission (F) of ECFP/Citrine (470/515 nm) or T-Sapphire/dimer2 (590/535 nm) was measured. The FRET (FECFP/Fcitrine or FT-Sapphire/Fdimer2) was then calculated. Data shown are concentration response curves with mean ± SEM of pEC50 and maximal response from four to nine experiments. *P < 0.05 vs. untargeted biosensors (one-way ANOVA with Tukey’s multiple comparisons test). (D) Representative confocal images of adult cardiomyocytes expressing AKAP18δ-red-cGES-DE5 or TnI-red-cGES-DE5 biosensors and immunolabelled against antibody against SERCA2 as described in ‘Methods’ section. Localization of the biosensors and SERCA2 is shown in the overlay and in the zoom. Increased contrast is applied to zoomed images. Scale bars: 10 μm. (E) Neonatal cardiomyocytes transfected with the indicated biosensors were lysed 24–48 h after transfection, and lysates were subjected to immunoprecipitation with anti-GFP. Direct interaction between AKAP18δ-red-cGES-DE5 biosensor and SERCA2, or TnI-red-cGES-DE5 biosensor and TnT was detected by immunoblotting with anti-SERCA2 and anti-TnT. Input is total cell lysates, and IP neg control is in the absence of anti-GFP. Shown is a representative of three independent experiments.