Abstract
Background
Current physical activity guidelines focus on volume and intensity for CVD prevention rather than common behaviors responsible for movement, including those for daily living activities. We examined the associations of a machine‐learned, accelerometer‐measured behavior termed daily life movement (DLM) with incident CVD.
Methods and Results
Older women (n=5416; mean age, 79±7 years; 33% Black, 17% Hispanic) in the Women’s Health Initiative OPACH (Objective Physical Activity and Cardiovascular Health) study without prior CVD wore ActiGraph GT3X+ accelerometers for up to 7 days from May 2012 to April 2014 and were followed for physician‐adjudicated incident CVD through February 28th, 2020 (n=616 events). DLM was defined as standing and moving in a confined space such as performing housework or gardening. Cox models estimated hazard ratios (HR) and 95% CI, adjusting for age, race and ethnicity, education, alcohol use, smoking, multimorbidity, self‐rated health, and physical function. Restricted cubic splines examined the linearity of the DLM‐CVD dose‐response association. We examined effect modification by age, body mass index, Reynolds Risk Score, and race and ethnicity. Adjusted HR (95% CIs) across DLM quartiles were: 1.00 (reference), 0.68 (0.55–0.84), 0.70 (0.56–0.87), and 0.57 (0.45–0.74); p‐trend<0.001. The HR (95% CI) for each 1‐hour increment in DLM was 0.86 (0.80–0.92) with evidence of a linear dose‐response association (p non‐linear>0.09). There was no evidence of effect modification by age, body mass index, Reynolds Risk Score, or race and ethnicity.
Conclusions
Higher DLM was independently associated with a lower risk of CVD in older women. Describing the beneficial associations of physical activity in terms of common behaviors could help older adults accumulate physical activity.
Keywords: aging, cardiovascular disease, epidemiology, lifestyle, machine learning, primary prevention
Subject Categories: Aging, Cardiovascular Disease, Epidemiology, Primary Prevention
Nonstandard Abbreviations and Acronyms
- CHAMPS
Community Health Activities Model Program for Seniors
- DLM
daily life movement
- LIPA
light intensity physical activity
- MV
moderate‐to‐vigorous intensity
- OPACH
Objective Physical Activity and Cardiovascular Health
- PA
physical activity
- WHI
Women’s Health Initiative
Clinical Perspective
What Is New?
Current physical activity guidelines focus on intensity for cardiovascular disease prevention rather than common behaviors underlying movement.
In older women, higher amounts of a machine‐learned accelerometer measured behavior of daily life movement, defined as standing and moving in confined space such as when performing housework or gardening, was associated with a lower risk of CVD.
The daily life movement‐cardiovascular disease association were generally consistent across age, body mass index, Reynolds Risk Score, and race and ethnicity, suggesting high generalizability.
What Are the Clinical Implications?
Describing the beneficial associations of physical activity in terms of common behaviors could help older adults accumulate physical activity.
Cardiovascular disease (CVD) continues to be the leading cause of death in the United States with rates highest in older adults, a rapidly growing demographic group. 1 , 2 , 3 In adults aged ≥85 years, the incidence of myocardial infarction (MI) and fatal coronary heart disease (CHD) is higher in women (125 per 1000) compared with men (90 per 1000). 3 The US 2018 Physical Activity Guidelines recommends at least 150 minutes/week of moderate‐to‐vigorous intensity (MV) PA for CVD prevention. 4 Studies of light intensity physical activity (LIPA) also suggest benefits against CVD. 4 , 5 , 6 The aerobic Physical Activity Guidelines emphasizes physical activity (PA) intensity rather than the behaviors producing the movements, including those involved in daily living activities. 4 Describing the health benefits of PA in terms of common behaviors that are accumulated during daily living activities could support efforts aimed at increasing PA in older adults.
Common behaviors may not be adequately captured in PA questionnaires, and commonly used accelerometer data processing methods (eg, cut points) focus on distinguishing movement intensity and do not yield information on the behavior’s underlying movement. 7 , 8 Machine learning algorithms have been developed and validated specifically for older adults to classify accelerometer data into behaviors. 8 , 9 The Two‐Level Behavior Classification algorithm classified accelerometer data into 1 of 5 behaviors: sitting, sitting in a vehicle, standing still, walking or running, or standing with ambulation. 8 In this algorithm, the standing with ambulation behavior consisted of walking in a confined space, included both light intensity and moderate‐to‐vigorous intensity movements, and encompassed daily life movements such as when performing household chores or gardening in an enclosed area, otherwise summarized as being “up and about”. In our previous study in the OPACH (Objective Physical Activity and Cardiovascular Health) cohort, more time spent in daily life movements that included standing with ambulation was associated with significantly lower risk of all‐cause mortality. 10 The associations of these daily life movements with incident CVD remains to be studied.
We applied a validated machine learning algorithm to analyze hip‐worn accelerometer data in community dwelling older women in the OPACH study. We examined (1) whether higher daily life movement (DLM) was associated with a lower risk of incident CVD, (2) the linearity of DLM‐CVD associations, and (3) effect modification by age, race and ethnicity, body mass index (BMI), and Reynolds Risk Score.
Methods
Study Population
The data that support the present study findings are available upon reasonable request from the Women’s Health Initiative (WHI) clinical coordinating center (https://sp.whi.org/researchers/data/WHIStudies/StudySites/AS286/Pages/home.aspx) in accordance with the WHI’s publications and presentations policies.
The WHI is a prospective study of postmenopausal women aged 50 to 79 years enrolled in the WHI Clinical Trial(s) or the Observational Study from 1993 to 1998 in 40 study sites. 11 The WHI OPACH ancillary study collected accelerometry data from 6489 ambulatory community‐dwelling women aged ≥63 years from May 2012 to April 2014 with follow‐up through February 28, 2020 for incident CVD. 12 Data were excluded from 611 women who returned accelerometers with insufficient data to apply the machine learning algorithm and 462 women with a history of MI or stroke at OPACH baseline, yielding an analytic sample of 5416 women. Study protocols were approved by the Fred Hutchinson Cancer Research Center and all women provided informed written consent.
CVD End Points
Major CVD was the primary end point that consisted of the first reported non‐fatal or fatal CHD, non‐fatal or fatal stroke, or death attributable to any CVD. CHD consisted of clinical MI, definite silent MI, or a death attributable to definite CHD or possible CHD. The first reported non‐fatal or fatal CHD, non‐fatal or fatal stroke, and CVD death were also examined as separate end points. Detailed descriptions of the WHI CVD adjudication process has been described. 12 , 13 Events were ascertained through review of mailed or telephone‐administered annual medical history questionnaires, and incident events were physician adjudicated following a review of participants’ medical records. 12 Kappa statistics for the inter‐rater agreement for CVD ascertainment ranged from 0.67 to 0.94. 13
PA and Behavior Classifications
PA was measured with the ActiGraph GT3X+ accelerometer, worn on the hip 24 hours/day except during risk of submersion in water (eg, bathing and swimming). ActiLife version 6 software aggregated the accelerometer data into 15 s epochs. The Choi algorithm identified periods of accelerometer non‐wear. 12 , 14 Sleep time was excluded using recorded in‐bed and out‐of‐bed times from sleep diaries. Missing sleep time was imputed using the participant’s average sleep time. The Community Health Activities Model Program for Seniors (CHAMPS) questionnaire assessed weekly frequency and duration for 41 physical activities endorsed by older adults. 15
Behaviors were determined using a validated machine‐learning algorithm published within the Two‐Level Behavior Classification package in R. 8 In an independent study, 39 older women wore a SenseCam camera around the neck for up to 7 days, with several images taken every minute. 8 Trained research assistants coded these images into 1 of 5 mutually exclusive behaviors: sitting (not in a vehicle), sitting in a vehicle, standing without ambulation, DLM, and walking or running. DLM encompassed time (hours/day) participants spent standing up while moving in a confined space. DLM included light and MV intensity movements involved in daily self‐care, walking around the kitchen, housework, and gardening. Annotated data from these images were used as ground truth data to train a random forest machine‐learning algorithm to classify hip‐worn accelerometer data into 1 of the 5 behaviors listed above. 8 The algorithm had a 66% sensitivity, 94% specificity, and a balanced accuracy of 79% for DLM compared with SenseCam images in the training sample and was validated in 2 other cohorts. 8 To evaluate whether intensity was important for incident CVD, DLM time was subsequently classified into sedentary time, light DLM, and MV DLM by applying accelerometer cut points derived specifically for older women in the OPACH Calibration Study (sedentary time was any 15‐s epoch with <19 vector magnitude counts, light DLM was any 15‐s epoch between 19 and 519 vector magnitude counts; and MV DLM was defined as any 15‐s epoch with ≥519 vector magnitude counts). 16 We calculated light and MV DLM metabolic equivalent (MET)‐hours/day by multiplying light and MV DLM hours/day by the median measured MET in the OPACH Calibration Study for light (2.0 METs) and MV (3.8 METs) activity. 16 Time spent in each intensity of DLM were averaged over all valid wear days. All DLM variables were adjusted for variation in awake wear time using the residuals method. 17
Covariates
Questionnaires ascertained participant age, self‐reported race and ethnicity (Black, White, Hispanic/Latina), education (≤high school equivalent, some college, college graduate), alcohol consumption (non‐drinker, <1 drink/week, ≥1 drink/week, unknown), current smoking status, self‐rated health (excellent/very good, good, poor/very poor), and walking assist device such as a cane or walker (never, occasionally, frequently or all the time). Physical function was ascertained using the Research And Development‐36 Health Survey, ranging from 0 to 100 with higher scores indicating higher physical functioning. 18 Trained study staff measured height and weight with a tape measure and calibrated bathroom scale, respectively, with BMI calculated as kg/m2. Systolic blood pressure was measured with an aneroid sphygmomanometer and the average of 2 measurements were recorded. We defined multimorbidity as the number of chronic health conditions (diabetes, hypertension, chronic obstructive pulmonary disease, osteoarthritis, depression, or history of falls), categorized as 0, 1, 2, or at least 3 conditions. Trained study staff obtained fasting (12 hours) blood from which serum glucose, high‐density lipoprotein cholesterol, and CRP (C‐reactive protein) were measured using standardized Clinical Laboratory Improvement Act‐approved methods at the University of Minnesota. 19 The Reynolds Risk Score, a summary measure of CVD risk, was calculated using age, systolic blood pressure, CRP, total and high‐density lipoprotein cholesterol, diabetes status, smoking status, and family history of MI. 20
Statistical Analysis
We performed statistical analyses in RStudio 1.3.1093 (https://rstudio.com/). We calculated means and SDs, or counts and proportions, for participant characteristics and compared these across DLM quartiles using F‐tests for continuous variables and Chi‐square tests for categorical variables.
To determine which self‐reported physical activities endorsed by older adults comprised DLM, simple linear regressions and r‐square correlations were computed between DLM time and total activity from all exercise‐related activities in the CHAMPS questionnaire and between DLM hours/day and each of the 41 activities contained in the CHAMPS questionnaire.
Multivariable Cox proportional hazards regression estimated HRs and 95% CIs for DLM hours/day quartile‐CVD end point associations. Follow‐up time was defined as the number of days from OPACH baseline to the first occurrence of CVD end point, or date of the last obtained annual medical update. Schoenfeld residuals were used to test the proportional hazards assumption; no violations were observed. Model 1 adjusted for age and race and ethnicity, as published studies in OPACH showed that PA and DLM vary by race and ethnicity. 6 , 10 Model 2 contained additional confounders: education, smoking status, alcohol consumption, physical function, multimorbidity, and self‐rated health. Model 3 contained model 2 covariates plus covariates considered mediators or confounders: BMI, systolic blood pressure, total cholesterol, high‐density lipoprotein, and CRP. Restricted cubic splines evaluated the linearity of the associations of total, light, and MV DLM with CVD end points in separate models, adjusting for model 2 covariates. For sensitivity analyses, we repeated these models with light and MV DLM mutually adjusted. To test whether intensity was important at the same amount of expenditure, we repeated our models with light and MV DLM MET‐hours/day using contrasts of regression coefficients. We performed stratified analyses by age (<80 years, ≥80 years), BMI (<30 kg/m2, ≥30 kg/m2), Reynolds Risk Score (<9.77 and ≥9.77, median split), and race and ethnicity (Black, White, Hispanic/Latina). We statistically evaluated effect modification with cross‐product interaction terms between continuous total DLM and stratification variables. Missing covariate data were imputed using multiple imputation by chained equations with the R mice package, including all variables for 5 iterations and 100 imputations. We set statistical significance to P<0.10 for effect modification and P<0.05 for all other tests.
Sensitivity Analyses
To evaluate potential reverse causality, we repeated the DLM quartile‐CVD end point models with major CVD occurring within the first 2 years of follow‐up excluded as a sensitivity analysis. To evaluate the possibility that walking assist device (eg, cane) use could impact mobility, which in turn could impact CVD and thus confound DLM‐CVD associations, we repeated the DLM‐quartile‐CVD end point models with walking assist device use added as a covariate.
Results
Study Population Characteristics
Women spent on average, 3.2 hours/day in DLM with an SD of 1.5 hours/day; 2.71 and 0.53 hours/day were spent in light and MV DLM, respectively (Table 1). Compared with women with DLM in the lowest quartile, women with DLM in the highest were younger, more likely to be Hispanic/Latina, similar in education, had a lower BMI, had higher physical function scores, and had more favorable CVD risk biomarker levels (Table 1). On average, of women’s awake time, ≈ 22% was spent in DLM, 64% was spent sitting, 6% was spent standing, 5% was spent riding in a vehicle, and 3% was spent walking or running (Figure 1).
Table 1.
Mean (SD) or Count (%) of OPACH (n=5416) Baseline (2012–2014) Sociodemographic and Health‐Related Characteristics
| Total | Quartiles of daily life movement (DLM) h/day | P value | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| <2.19 | 2.19–3.09 | 3.09–4.11 | ≥4.11 | |||
| Age, y | 78.5 (6.7) | 80.4 (6.7) | 78.8 (6.7) | 77.9 (6.5) | 76.7 (6.3) | <0.001 |
| Race and ethnicity | ||||||
| White | 2664 (49.2) | 755 (55.8) | 677 (50.0) | 652 (48.2) | 580 (42.8) | <0.001 |
| Black | 1798 (33.2) | 459 (33.9) | 487 (36.0) | 423 (31.2) | 429 (31.7) | |
| Hispanic/Latina | 954 (17.6) | 140 (10.3) | 190 (14.0) | 279 (20.6) | 345 (25.5) | |
| Highest education level | ||||||
| High school or less | 1079 (20.0) | 234 (17.4) | 268 (20.0) | 276 (20.6) | 301 (22.3) | 0.01 |
| Some college | 2068 (38.4) | 541 (40.2) | 484 (36.0) | 537 (40.0) | 506 (37.5) | |
| College graduate | 2237 (41.5) | 572 (42.5) | 591 (44.0) | 530 (39.5) | 544 (40.3) | |
| Health behavior/status, n (%) | ||||||
| Current smoker | 134 (2.5) | 51 (3.8) | 31 (2.3) | 24 (1.8) | 28 (2.1) | 0.004 |
| Alcohol in past 3 mo | ||||||
| Non‐drinker | 1808 (33.4) | 526 (38.8) | 458 (33.8) | 410 (30.3) | 414 (30.6) | <0.001 |
| <1 drink/wk | 1735 (32.0) | 430 (31.8) | 460 (34.0) | 425 (31.4) | 420 (31.0) | |
| ≥1 drink/wk | 1435 (26.5) | 263 (19.4) | 319 (23.6) | 432 (31.9) | 421 (31.1) | |
| Unknown | 438 (8.1) | 135 (10.0) | 117 (8.6) | 87 (6.4) | 99 (7.3) | |
| BMI, kg/m2 | 28.1 (5.7) | 30.1 (6.4) | 28.6 (5.5) | 27.5 (5.3) | 26.1 (4.8) | <0.001 |
| Self‐rated health | ||||||
| Excellent or very good | 2829 (52.4) | 543 (40.3) | 694 (51.3) | 773 (57.3) | 819 (60.8) | <0.001 |
| Good | 2095 (38.8) | 612 (45.4) | 548 (40.5) | 485 (35.9) | 450 (33.4) | |
| Fair or poor | 474 (8.8) | 193 (14.3) | 110 (8.1) | 92 (6.8) | 79 (5.9) | |
| RAND‐36 physical function score | 70.0 (25.5) | 56.7 (27.8) | 69.2 (24.9) | 74.5 (22.5) | 79.6 (20.3) | <0.001 |
| Chronic health conditions | ||||||
| Diabetes | 1042 (19.2) | 356 (26.3) | 263 (19.4) | 249 (18.4) | 174 (12.9) | <0.001 |
| Hypertension | 3813 (70.4) | 1049 (77.5) | 993 (73.3) | 939 (69.4) | 832 (61.4) | <0.001 |
| COPD | 159 (2.9) | 55 (4.1) | 37 (2.7) | 35 (2.6) | 32 (2.4) | 0.04 |
| Osteoarthritis | 2956 (54.6) | 756 (55.8) | 747 (55.2) | 715 (52.8) | 738 (54.5) | 0.43 |
| Depression | 421 (7.8) | 124 (9.2) | 109 (8.1) | 92 (6.8) | 96 (7.1) | 0.09 |
| Falls in past 12 mo | 1546 (29.5) | 411 (31.4) | 391 (29.9) | 368 (28.1) | 376 (28.6) | 0.24 |
| Multimorbidity count | ||||||
| 0 conditions | 1850 (34.2) | 423 (31.2) | 445 (32.9) | 492 (36.3) | 490 (36.2) | <0.001 |
| 1 condition | 2578 (47.6) | 626 (46.2) | 658 (48.6) | 639 (47.2) | 655 (48.4) | |
| 2 conditions | 842 (15.5) | 246 (18.2) | 215 (15.9) | 196 (14.5) | 185 (13.7) | |
| ≥3 conditions | 146 (2.7) | 59 (4.4) | 36 (2.7) | 27 (2.0) | 24 (1.8) | |
| Walking assist device (eg, cane) use | ||||||
| Never | 3739 (71.0) | 676 (51.4) | 921 (70.0) | 1033 (78.4) | 1109 (83.8) | <0.001 |
| Occasionally | 849 (16.1) | 259 (19.7) | 238 (18.1) | 187 (14.2) | 165 (12.5) | |
| Frequently or all the time | 681 (12.9) | 379 (28.8) | 156 (11.9) | 97 (7.4) | 49 (3.7) | |
| CVD biomarkers, mean (SD) | ||||||
| Reynolds Risk Score* | 13.3 (11.4) | 18.2 (14.2) | 13.9 (11.0) | 12.0 (9.8) | 9.5 (8.3) | <0.001 |
| Systolic blood pressure, mm Hg | 125.6 (14.1) | 127.7 (15.2) | 126.3 (14.3) | 125.1 (13.6) | 123.6 (13.2) | <0.001 |
| Log hs‐CRP, mg/L | 0.62 (1.05) | 0.82 (1.09) | 0.71 (1.05) | 0.59 (1.04) | 0.39 (0.95) | <0.001 |
| Total cholesterol, mg/dL | 198.9 (38.9) | 193.8 (40.2) | 197.1 (40.1) | 199.3 (36.9) | 204.7 (37.7) | <0.001 |
| HDL, mg/dL | 60.8 (14.9) | 57.8 (14.6) | 59.7 (14.3) | 61.2 (14.5) | 64.3 (15.4) | <0.001 |
| Daily life movement † , mean (SD) | ||||||
| Total DLM, h/day † | 3.2 (1.5) | 1.5 (0.5) | 2.6 (0.3) | 3.6 (0.3) | 5.2 (0.9) | <0.001 |
| Light intensity DLM, hours/day † | 2.7 (1.2) | 1.3 (0.5) | 2.3 (0.3) | 3.0 (0.4) | 4.2 (0.8) | <0.001 |
| Moderate‐to‐vigorous intensity DLM † , hours/day † | 0.5 (0.4) | 0.2 (0.1) | 0.4 (0.2) | 0.6 (0.3) | 1.0 (0.5) | <0.001 |
| Follow‐up, mean (SD) | ||||||
| Major CVD, y | 6.0 (1.6) | 5.5 (1.8) | 6.0 (1.5) | 6.1 (1.5) | 6.3 (1.3) | <0.001 |
| CHD, y | 6.0 (1.5) | 5.6 (1.7) | 6.1 (1.4) | 6.2 (1.4) | 6.3 (1.2) | <0.001 |
| Stroke, y | 6.0 (1.5) | 5.6 (1.8) | 6.1 (1.4) | 6.2 (1.4) | 6.4 (1.2) | <0.001 |
| CVD death, y | 6.1 (1.4) | 5.7 (1.7) | 6.2 (1.3) | 6.2 (1.3) | 6.4 (1.1) | <0.001 |
BMI indicates body mass index; RAND, Research And Development; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DLM, daily life movement; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; OPACH, Objective Physical Activity and Cardiovascular Health.
P value: Chi‐square test for categorical variables and F‐test for continuous variables.
Ten‐year predicted probability (%) of a clinical cardiovascular disease event.
All physical activity related variables are adjusted for accelerometer awake wear time using the residuals method.
Figure 1. Average awake time spent across 5 behaviors (sitting, sitting in a vehicle, standing, walking, or running, and daily life movement) and average daily life movement time spent across sedentary behavior, light intensity, and moderate‐to‐vigorous intensity.
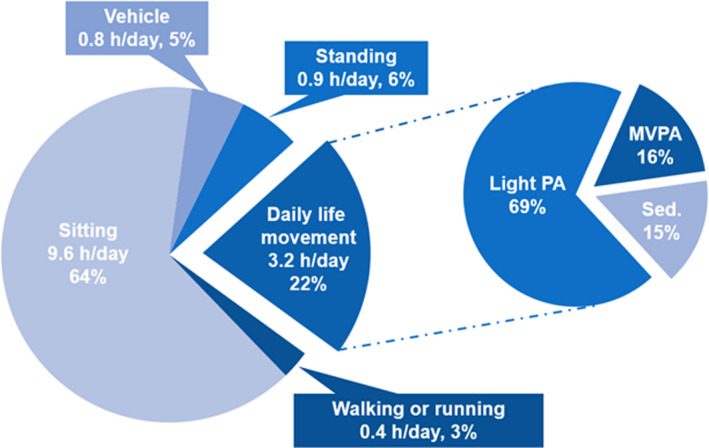
MVPA indicates moderate‐to‐vigorous intensity physical activity; and PA, physical activity.
The Pearson correlation between DLM time and total activity estimated from all 41 activities in the CHAMPS questionnaire was 0.25. Of the 41 activities covered in the CHAMPS questionnaire, gardening and housework were most strongly positively correlated with total DLM (correlation >0.23; Figure 2). Computer use and reading were most strongly negatively correlated (correlation <−0.06) with total DLM (Figure 2).
Figure 2. Correlations from linear regression of total daily life movement on 41 activities in Community Health Activities Model Program for Seniors questionnaire.
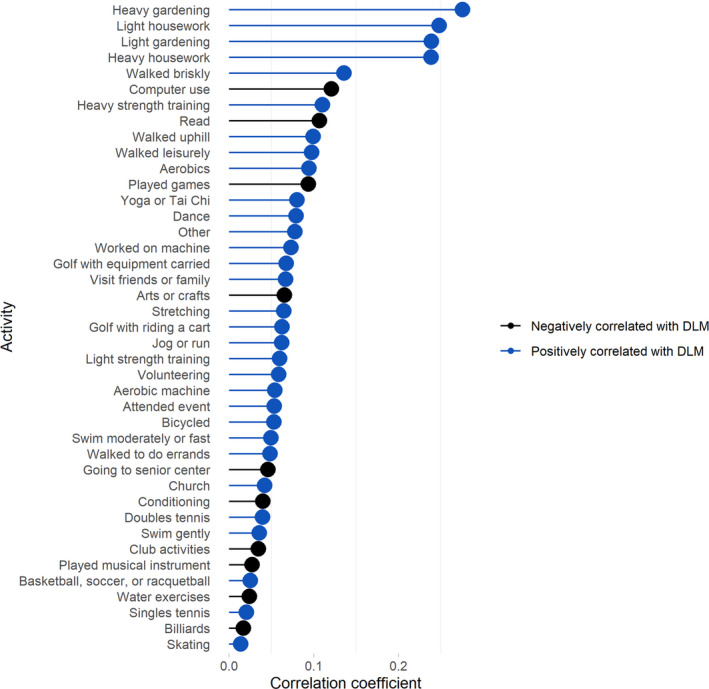
DLM indicates daily life movement.
Association of DLM Quartiles With Incident Major CVD, CHD, Stroke, and CVD Death
The median follow‐up was ≈ 6.5 years and there were 616 major CVD events, 268 CHD events, 253 stroke events, and 331 CVD deaths. Unadjusted incidence rates for CVD end points were lower across higher quartiles of DLM (Table 2). After adjusting for potential confounders in model 2, the HR (95% CI; p‐trend) comparing women with DLM in the highest quartile to those in the lowest quartile was 0.57 (0.45–0.74; <0.001) for major CVD, 0.57 (0.38–0.84; 0.003) for CHD, 0.70 (0.47–1.03; 0.02) for stroke, and 0.38 (0.26–0.56; <0.001) for CVD death (Table 2). Associations remained consistent in magnitude, direction, and statistical significance after adjustment for potential mediators and confounders in model 3 and for complete case analysis (Table 2 and Table S1). Results from sensitivity analyses evaluating reverse causation where data were excluded from 163 women with incident major CVD that occurred within the first 2 years of follow‐up were generally consistent in direction, magnitude, and significance to those from the main analyses (Table S2). In this sensitivity analysis, the HR (95% CI; p‐trend) for stroke comparing women with DLM in the highest quartile to those with DLM in the lowest in model 2 changed to 0.84 (0.54–1.31; 0.09; Table S2). Additional adjustment for walking assist device use did not appreciably change results (Table S1).
Table 2.
Associations of Daily Life Movement Quartiles With Incident Major CVD, CHD, Stroke, and CVD Death in the OPACH Cohort (n=5416) 2012 to 2020
| Quartiles of total daily life movement h/day | p‐trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| <2.19 | 2.19–3.09 | 3.09–4.11 | ≥4.11 | ||
| Major CVD | |||||
| Events (rate*) | 240 (32.3) | 147 (18.2) | 132 (16) | 97 (11.4) | |
| Model 1 † , ‡ , § | 1 (ref) | 0.63 (0.51–0.77) | 0.61 (0.49–0.76) | 0.48 (0.38–0.62) | <0.001 |
| Model 2 ‡ , § , ¶ | 1 (ref) | 0.68 (0.55–0.84) | 0.70 (0.56–0.87) | 0.57 (0.45–0.74) | <0.001 |
| Model 3 ‡ , § , # | 1 (ref) | 0.69 (0.56–0.85) | 0.72 (0.57–0.90) | 0.60 (0.47–0.78) | <0.001 |
| CHD | |||||
| Events (rate*) | 108 (14.3) | 59 (7.2) | 62 (7.4) | 39 (4.5) | |
| Model 1 † , ‡ , § | 1 (ref) | 0.56 (0.41–0.77) | 0.63 (0.46–0.87) | 0.43 (0.29–0.63) | <0.001 |
| Model 2 ‡ , § , ¶ | 1 (ref) | 0.64 (0.46–0.88) | 0.80 (0.57–1.11) | 0.57 (0.38–0.84) | 0.003 |
| Model 3 ‡ , § , # | 1 (ref) | 0.65 (0.47–0.90) | 0.83 (0.59–1.16) | 0.61 (0.40–0.92) | 0.01 |
| Stroke | |||||
| Events (rate*) | 77 (10.2) | 71 (8.7) | 58 (6.9) | 47 (5.5) | |
| Model 1 † , ‡ , § | 1 (ref) | 0.93 (0.67–1.29) | 0.81 (0.58–1.15) | 0.70 (0.48–1.02) | 0.02 |
| Model 2 ‡ , § , ¶ | 1 (ref) | 0.95 (0.68–1.31) | 0.81 (0.56–1.15) | 0.70 (0.47–1.03) | 0.02 |
| Model 3 ‡ , § , # | 1 (ref) | 0.95 (0.68–1.32) | 0.82 (0.57–1.17) | 0.71 (0.48–1.06) | 0.03 |
| CVD death | |||||
| Events (rate*) | 164 (21.3) | 67 (8) | 62 (7.3) | 38 (4.4) | |
| Model 1 † , ‡ , § | 1 (ref) | 0.43 (0.32–0.57) | 0.46 (0.34–0.61) | 0.31 (0.22–0.44) | <0.001 |
| Model 2 ‡ , § , ¶ | 1 (ref) | 0.47 (0.35–0.62) | 0.55 (0.40–0.75) | 0.38 (0.26–0.56) | <0.001 |
| Model 3 ‡ , § , # | 1 (ref) | 0.47 (0.35–0.63) | 0.56 (0.41–0.76) | 0.39 (0.27–0.58) | <0.001 |
Major cardiovascular disease represents the composite of the first reported fatal or non‐fatal coronary heart disease, fatal or non‐fatal stroke, or death attributable to any cardiovascular disease. The first reported fatal or non‐fatal coronary heart disease, fatal or non‐fatal stroke, and death attributable to any cardiovascular disease were examined as separate end points.
CHD indicates coronary heart disease; CVD, cardiovascular disease; OPACH, Objective Physical Activity and Cardiovascular Health; Q1‒Q4, quartile 1 to quartile 4; and RAND, Research And Development.
Event rate per 1000 person‐years.
Model 1 adjusted for age and race and ethnicity.
Data are hazard ratio (95% CI).
Model results estimated using multiple imputation by chained equations from R mice package.
Model 2 adjusted for model 1 covariates plus education, alcohol intake, smoking status, multimorbidity, self‐rated health, and RAND‐36 physical function score.
Model 3 adjusted for model 2 covariates plus body mass index, systolic blood pressure, total cholesterol, high‐density lipoprotein, and high‐sensitivity C‐reactive protein.
Continuous Dose‐Response Associations of DLM With Incident Major CVD, CHD, Stroke, and CVD Death
To determine if intensity was important for DLM‐CVD associations, we evaluated the linearity of the associations of total, light, and MV DLM with CVD end points. Dose‐response trajectories were all linear (all p‐non‐linear >0.09), so DLM was modeled in continuous linear functional form (Figure 3 and Table S3; in Figure S1 and Table S4, light and MV intensity daily life movement were mutually adjusted).
Figure 3. Continuous dose‐response associations of total, light intensity, and moderate‐to‐vigorous intensity daily life movement hours/day quantiles with (A) incident major cardiovascular disease, (B) coronary heart disease, (C) stroke, and (D) cardiovascular disease death.
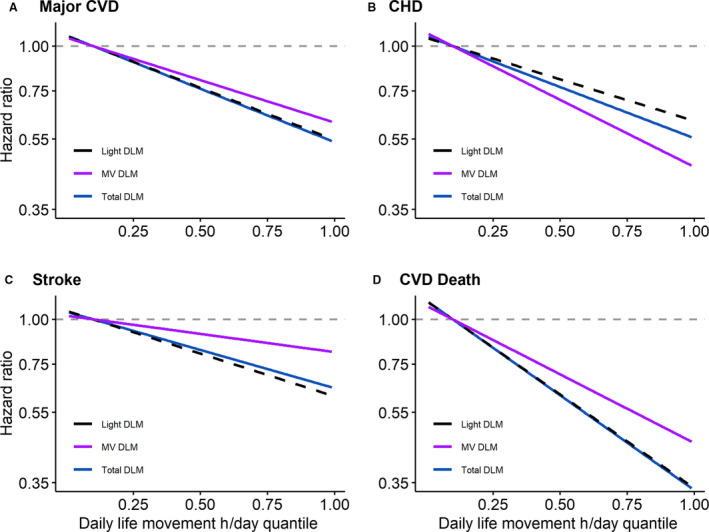
Models adjusted for age, race and ethnicity, education, alcohol intake, smoking status, multimorbidity, self‐rated health, and RAND‐36 physical function score (n=5325). Results were trimmed at the 1st and 99th percentiles. The reference was set to the 10th percentile of total (1.52 hours), light intensity (1.31 hours), or moderate‐to‐vigorous intensity (0.12 hours or 7.3 minutes) daily life movement. Hazard ratios (95% CI) for associations of total, light intensity, and moderate‐to‐vigorous intensity daily life movement at 5th, 25th, 50th, 75th, and 95th percentiles with CVD are displayed in Table S3. CVD indicates cardiovascular disease; CHD, coronary heart disease; DLM, daily life movement; MV, moderate‐to‐vigorous intensity; and RAND, Research And Development.
After adjustment in model 2, the HR (95% CI) for each 1‐hour/day increment in total DLM was 0.86 (0.80–0.92) for major CVD, 0.86 (0.77–0.95) for CHD, 0.89 (0.81–0.99) for stroke, and 0.76 (0.69–0.84) for CVD death (Table S5). The HR (95% CI) for each 1‐hour/day increment in light DLM was 0.84 (0.77–0.91) for major CVD, 0.85 (0.76–0.96) for CHD, 0.87 (0.77–0.98) for stroke, and 0.72 (0.64–0.81) for CVD death. The HR (95% CI) for each 1‐hour/day increment in MV DLM was 0.67 (0.51–0.86) for major CVD, 0.50 (0.33–0.77) for CHD, 0.82 (0.57–1.19) for stroke, and 0.51 (0.34–0.75) for CVD death. In mutually adjusted models, results for light DLM were consistent in magnitude and significance except for CHD, which was attenuated to non‐significance while results for MV DLM were attenuated to non‐significance except for CHD, which remained significant (Table S6). We examined mutually adjusted associations of light and MV DLM MET‐hours/day with CVD end points to determine if intensity is important at the same amount of energy expenditure (Table S7). Contrasts between regression coefficients comparing MV and light DLM MET‐hours/day were not significant (all P>0.09).
Effect Modification of Associations of DLM With Major CVD, CHD, Stroke, and CVD Death
In stratified analyses, point estimates suggested that each 1‐hour increment in total DLM was associated with a lower risk of CVD end points for all subgroups except for CHD; we observed evidence of effect modification by age (p‐interaction=0.08) for CHD (Table 3). Each 1‐hour increment in total DLM was associated with a lower risk of CHD for women aged ≥80 years (HR, 0.79; 95% CI, 0.69–0.89) but not for women aged <80 years (HR, 1.02; 95% CI, 0.86–1.22). Otherwise, there was no evidence of effect modification by age, BMI, Reynolds Risk Score, or race and ethnicity for total DLM‐CVD end point associations (all P>0.10).
Table 3.
Associations of a 1‐Hour Increment in Total Daily Life Movement With CVD End Points Stratified by Baseline Characteristics in OPACH (2012–2020)
| n | Major CVD | CHD | Stroke | CVD death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n events | HR (95% CI) | p‐interaction | n events | HR (95% CI) | p‐interaction | n events | HR (95% CI) | p‐interaction | n events | HR (95% CI) | p‐interaction | ||
| Overall | 5416 | 616 | 0.86 (0.80–0.91) | 268 | 0.86 (0.78–0.95) | 253 | 0.89 (0.80–0.98) | 331 | 0.75 (0.68–0.83) | ||||
| Age | 0.11 | 0.08 | 0.79 | 0.43 | |||||||||
| <80 y | 2799 | 172 | 0.94 (0.84–1.05) | 70 | 1.02 (0.86–1.22) | 85 | 0.85 (0.72–1.00) | 61 | 0.90 (0.74–1.10) | ||||
| ≥80 y | 2617 | 444 | 0.82 (0.75–0.89) | 198 | 0.79 (0.69–0.89) | 168 | 0.90 (0.80–1.02) | 270 | 0.72 (0.64–0.80) | ||||
| BMI | 0.54 | 0.47 | 0.50 | 0.26 | |||||||||
| <30 kg/m2 | 3736 | 421 | 0.85 (0.78–0.92) | 174 | 0.84 (0.74–0.95) | 180 | 0.89 (0.79–1.00) | 223 | 0.73 (0.65–0.82) | ||||
| ≥30 kg/m2 | 1680 | 195 | 0.86 (0.75–0.98) | 94 | 0.91 (0.75–1.11) | 73 | 0.86 (0.69–1.07) | 108 | 0.81 (0.66–0.98) | ||||
| Reynolds Risk Score | 0.60 | 0.65 | 0.51 | 0.81 | |||||||||
| <9.77 | 2685 | 173 | 0.93 (0.82–1.04) | 59 | 0.96 (0.79–1.17) | 92 | 0.90 (0.76–1.06) | 76 | 0.91 (0.76–1.09) | ||||
| ≥9.77 | 2731 | 443 | 0.84 (0.77–0.91) | 209 | 0.86 (0.76–0.97) | 161 | 0.89 (0.77–1.01) | 255 | 0.71 (0.63–0.80) | ||||
| Race and ethnicity | 0.93 | 0.98 | 0.77 | 0.81 | |||||||||
| White | 2664 | 409 | 0.85 (0.78–0.93) | 179 | 0.85 (0.75–0.97) | 155 | 0.89 (0.78–1.02) | 239 | 0.75 (0.67–0.84) | ||||
| Black | 1798 | 149 | 0.84 (0.74–0.96) | 59 | 0.90 (0.73–1.11) | 71 | 0.82 (0.68–1.00) | 71 | 0.71 (0.58–0.87) | ||||
| Hispanic/Latina | 954 | 58 | 0.90 (0.74–1.10) | 30 | 0.84 (0.62–1.14) | 27 | 0.97 (0.72–1.32) | 21 | 0.91 (0.60–1.37) | ||||
Major cardiovascular disease represents the composite of the first reported fatal or non‐fatal coronary heart disease, fatal or non‐fatal stroke, or death attributable to any cardiovascular disease. The first reported fatal or non‐fatal coronary heart disease, fatal or non‐fatal stroke, and death attributable to any cardiovascular disease were examined as separate end points.
Models adjusted for age, race and ethnicity, educational attainment, smoking status, alcohol use, multimorbidity, RAND‐36 physical function score, and self‐rated health. Model results estimated using multiple imputation by chained equations from R mice package.
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; OPACH, Objective Physical Activity and Cardiovascular Health; and RAND, Research And Development.
Discussion
Main Findings
In this racially and ethnically diverse prospective study of older women, higher amounts of DLM were independently associated with lower risk of incident CVD. Compared with women with <2 hours/day of DLM, those with at least 4 hours/day had a 43% lower risk of major CVD, 43% lower risk of CHD, 30% lower risk of stroke, and notably, 62% lower risk of CVD death. There were significant inverse linear dose‐response associations between total DLM and CVD end points, indicating that higher DLM could contribute towards CVD prevention regardless of where one falls on the distribution. We examined whether DLM intensity was important as ≈ 16% of DLM is MV. Higher amounts of light DLM were associated with lower risk of major CVD, stroke, and CVD death, and higher amounts of MV‐DLM were associated with lower CHD risk. However, light DLM‐CVD end point associations were not significantly different to those for MV DLM at the same amount of energy expenditure, suggesting higher amounts of both light and MV DLM are similarly associated with lower risk of CVD end points. DLM‐CVD end point associations were generally consistent across age, race and ethnicity, BMI, and Reynolds Risk Score, suggesting high generalizability that DLM could contribute towards CVD prevention among older women. Overall, the present study shows that DLM, a behavior summarized as being “up and about”, is important for CVD prevention in older women.
Our results are noteworthy since much of the movement engaged in by older adults is associated with daily life tasks, which may not be considered PA by older adults themselves or by questionnaires. 21 Older adults may have difficulty engaging in MV PA because of barriers such as age‐related changes in physical function and uncertainty about options for PA. 16 , 22 , 23 , 24 Describing the health benefits of PA in terms of common behaviors such as DLM could reduce these barriers and help older adults accumulate movement. DLM can be done in the home setting, which could be more accessible than MV PA or common behaviors such as walking where physical environmental factors such as sidewalks could influence participation. 23 Thus, approaches to studying behaviors underlying PA could be an important component in efforts aimed at increasing movement in older adults.
Our study extends the literature on the prospective associations of machine‐learned accelerometer‐measured behaviors with health outcomes. To our knowledge, ours is the first study to examine such associations with incident CVD and with CHD and stroke as separate end points. Our previous study in OPACH observed that compared with women with <2.1 hours/day of DLM (labeled standing with ambulation), those with at least 4.1 hours/day of DLM had a 50% lower risk of all‐cause mortality. 10 The focus of the present study was an in‐depth examination of the associations of DLM with non‐fatal and fatal CVD events in a composite as well as separate CVD‐related end points. Another study in OPACH observed that compared with women with <3.9 hours/day of LIPA, those with at least 5.6 hours/day had a 42% and 22% lower risk of CHD and CVD, respectively, consistent in direction to results in the present study. 6 The present study incorporated data from an additional 3 years of follow‐up and focused on the common behavior of DLM rather than focusing specifically on the PA intensity of accelerometer counts, which do not yield information on the behaviors producing the movements. DLM is strongly negatively correlated with sedentary time (r=−0.87), strongly positively correlated with LIPA (r=0.78), and moderately positively correlated with MV PA (r=0.65) in OPACH.
As of this writing, relatively few studies examined objectively measured LIPA‐CVD risk factor associations, and findings are mixed. A published study in OPACH observed favorable associations of accelerometer‐measured LIPA with high‐density lipoprotein, glucose, and CRP. 19 A systematic review of intervention studies observed that LIPA improved blood pressure in sedentary individuals with hypertension and diabetes, but not for lipid profiles. 25 Muscle activation during DLM could induce changes in heart rate, blood pressure, and vascular tone, leading to increased energy expenditure and improvements in endothelial function and lipid metabolism. 4 , 26 , 27 Additional research on accelerometer‐measured LIPA‐CVD risk factor associations across a wider age range and in men is warranted.
Strengths and Limitations
The present study has notable strengths. OPACH is a large and racially and ethnically diverse study population of older women. A unique strength was the machine‐learned algorithm used to classify accelerometer data into behaviors. Sufficient follow‐up time has accumulated to examine CHD, stroke, and CVD death separately. We adjusted for differences in health status that can affect the ability to move, including self‐rated health, multimorbidity, and physical function. DLM was robustly associated with CVD end points after extensive adjustment for confounders and risk factors and results were consistent across subgroups. Evidence from sensitivity analyses that excluded data from 163 women who had incident major CVD within the first 2 years of follow‐up suggest that DLM‐CVD associations are not explained by reverse causation bias as results were generally consistent in direction and magnitude to those from the quartile DLM analyses. Results for stroke in this sensitivity analysis were consistent in direction to those from the quartile DLM analyses and the weaker HRs, wider 95% CIs, and non‐significant p‐trend could be attributable to a loss in statistical power. We addressed missing data by using multiple imputation by chained equations to reduce selection bias and enhance the precision of study results.
The present study has limitations. Women wore accelerometers for up to 7 days, which might not fully capture DLM for all women. The gold standard of direct observation for behavior measurement is not available in OPACH, as it is challenging to collect in large free‐living populations. Approximately 28% of DLM was misclassified as standing still or walking in the training sample. 8 However, an external validation conducted on the Two‐Level Behavior Classification algorithm determined that its performance was not significantly related to age, gait speed, use of a walking aid, falls, or physical functioning measured with the Short Physical Performance Battery, suggesting that the algorithm is robust across these characteristics. 8 Additionally, since the accelerometer data were collected before CVD onset, DLM misclassification is likely unrelated to the outcome and therefore DLM measurement error is likely nondifferential, yielding results that would be biased towards the null. However, it is reassuring that the self‐reported physical activities most strongly correlated with DLM were housework and gardening, consistent with the behaviors the algorithm was trained to classify. Measurement error from the Two‐Level Behavior Classification algorithm and known measurement error from applying accelerometer cut‐points from the OPACH Calibration Study resulted in 15% of DLM time being classified as sedentary time. 8 , 16 Despite this error, the DLM behavioral compartment was more strongly associated with CVD than when using accelerometer cut‐points in a published OPACH study. 6 Applying absolute cut‐points to classify DLM intensity to examine whether DLM intensity is important also assumed that women experienced the same intensity with accelerometer counts in the specified ranges. Although we applied calibrated cut‐points from a laboratory study of 200 OPACH women, future studies should consider developing methods to examine intensity levels relative to the maximum exertion that participants are capable of, to investigate intensity on a relative scale. Lastly, given a study population of older women in OPACH, replication in men and younger individuals to increase generalizability is warranted.
Conclusions
The present study showed that higher amounts of DLM, summarized as “being up and about”, is associated with a lower risk of major CVD, CHD, stroke, and CVD death in community‐living older women, suggesting that “all movement counts” towards CVD prevention. Replication in other cohorts and in men, in addition to large randomized trials including the WHISH (WHI Strong and Healthy) trial, are needed to confirm these findings. 28 Nonetheless, DLM should be promoted given its ubiquity in everyday life and relatively low risk. To determine the scope of potential health benefits of DLM, future research should test associations with other aging‐related outcomes. Healthcare providers and future national physical guidelines should consider describing the health benefits of PA in terms of common behaviors resulting in PA, such as DLM, which could help older adults accumulate PA.
Sources of Funding
This work was supported by the National Institute on Aging (P01 AG052352 to A.Z.L.) and the National Heart, Lung, and Blood Institute who provided funding for the OPACH study (grant number R01 HL105065 to A.Z.L.). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. Funding also came from a training grant provided by the National Institute on Aging (grant number 5T32AG058529‐03 to SN). All funding agencies were not involved in the design of the study, data collection, analysis, data interpretation, or manuscript writing.
Disclosures
None.
Supporting information
Tables S1–S7
Figure S1
Acknowledgments
We thank the WHI participants, staff, and investigators. The short list of WHI investigators can be found at: https://www‐whi‐org.s3.us‐west‐2.amazonaws.com/wp‐content/uploads/WHI‐Investigator‐Short‐List.pdf. The full list of WHI Investigators can be found at the following site: https://www.whi.org/doc/WHI‐Investigator‐Long‐List.pdf.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023433
For Sources of Funding and Disclosures, see page 12.
References
- 1. Xu J, Murphy S, Kochanek K, Arias E. Mortality in the United States. NCHS Data Brief. 2018;355:2020. [PubMed] [Google Scholar]
- 2. Colby S, Ortman J. Projections of the size and composition of the US population: 2014 to 2060: Population estimates and projections, the United States Census Bureau. 2017. [Google Scholar]
- 3. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update: a report from the American heart association. Circulation. 2021;143:254–743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 4. Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: 2018. [Google Scholar]
- 5. Rees‐Punia E, Evans EM, Schmidt MD, Gay JL, Matthews CE, Gapstur SM, Patel AV. Mortality risk reductions for replacing sedentary time with physical activities. Am J Prev Med. 2019;56:736–741. doi: 10.1016/j.amepre.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LaCroix AZ, Bellettiere J, Rillamas‐Sun E, Di C, Evenson KR, Lewis CE, Buchner DM, Stefanick ML, Lee I‐M, Rosenberg DE, et al. Association of light physical activity measured by accelerometry and the incidence of coronary heart disease and cardiovascular disease in older women. JAMA Open. 2019;2:e190419. doi: 10.1001/jamanetworkopen.2019.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doherty AR, Kelly P, Kerr J, Marshall S, Oliver M, Badland H, Hamilton A, Foster C. Using wearable cameras to categorise type and context of accelerometer‐identified episodes of physical activity. Int J Behav Nutr Phys Act. 2013;10:22. doi: 10.1186/1479-5868-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg D, Godbole S, Ellis K, Di C, Lacroix A, Natarajan L, Kerr J. Classifiers for accelerometer‐measured behaviors in older women. Med Sci Sports Exerc. 2017;49:610–616. doi: 10.1249/MSS.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kerr J, Carlson J, Godbole S, Cadmus‐Bertram L, Bellettiere J, Hartman S. Improving hip‐worn accelerometer estimates of sitting using machine learning methods. Med Sci Sports Exerc. 2018;50:1518–1524. doi: 10.1249/MSS.0000000000001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain P, Bellettiere J, Glass N, LaMonte MJ, Di C, Wild RA, Evenson KR, LaCroix AZ. The relationship of accelerometer‐assessed standing time with and without ambulation and mortality: the WHI OPACH study. Journals Gerontol Ser A. 2021;76:77–84. doi: 10.1093/gerona/glaa227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, Kuller L, Manson J, Oberman A, Prentice RL, et al. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 12. LaCroix AZ, Rillamas‐Sun E, Buchner D, Evenson KR, Di C, Lee I‐M, Marshall S, LaMonte MJ, Hunt J, Tinker LF, et al. The objective physical activity and cardiovascular disease health in older women (OPACH) study. BMC Public Health. 2017;17:1–12. doi: 10.1186/s12889-017-4065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self‐report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314 [DOI] [PubMed] [Google Scholar]
- 14. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 16. Evenson KR, Wen F, Herring AH, Di C, LaMonte MJ, Tinker LF, Lee IM, Rillamas‐Sun E, LaCroix AZ, Buchner DM. Calibrating physical activity intensity for hip‐worn accelerometry in women age 60 to 91 years: the Women’s Health Initiative OPACH Calibration Study. Prev Med Reports. 2015;2:750–756. doi: 10.1016/j.pmedr.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 18. Hays RD, Sherbourne CD, Mazel RM. The rand 36‐item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 19. LaMonte MJ, Lewis CE, Buchner DM, Evenson KR, Rillamas‐Sun E, Di C, Lee I‐M, Bellettiere J, Stefanick ML, Eaton CB, et al. Both light intensity and moderate‐to‐vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: the objective physical activity and cardiovascular health (OPACH) study. J Am Heart Assoc. 2017;6:e007064. doi: 10.1161/JAHA.117.007064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, Rossouw JE, Wassertheil‐Smoller S, Ridker PM. Comparison of the framingham and reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation. 2012;125:1748–1756. doi: 10.1161/CIRCULATIONAHA.111.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colbert LH, Matthews CE, Schoeller DA, Havighurst TC, Kim KM. Intensity of physical activity in the energy expenditure of older Adults. J Aging Phys Act. 2014;22:571–577. doi: 10.1123/JAPA.2012-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerr J, Rosenberg DE, Nathan A, Millstein RA, Carlson JA, Crist K, Wasilenko K, Bolling K, Castro CM, Buchner DM, et al. Applying the ecological model of behavior change to a physical activity trial in retirement communities: Description of the study protocol. Contemp Clin Trials. 2012;33:1180–1188. doi: 10.1016/j.cct.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bethancourt HJ, Rosenberg DE, Beatty T, Arterburn DE. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10–20. doi: 10.3121/cmr.2013.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Floegel TA, Giacobbi PR, Dzierzewski JM, Aiken‐Morgan AT, Roberts B, McCrae CS, Marsiske M, Buman MP. Intervention markers of physical activity maintenance in older adults. Am J Health Behav. 2015;39:487–499. doi: 10.5993/AJHB.39.4.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batacan RB, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of light intensity activity on CVD risk factors: a systematic review of intervention studies. Biomed Res Int. 2015;2015:596367. doi: 10.1155/2015/596367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merino J, Ferré R, Girona J, Aguas D, Cabré A, Plana N, Vinuesa A, Ibarretxe D, Basora J, Buixadera C, et al. Even low physical activity levels improve vascular function in overweight and obese postmenopausal women. Menopause. 2013;20:1036–1042. doi: 10.1097/GME.0b013e31828501c9 [DOI] [PubMed] [Google Scholar]
- 27. Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72:1622–1639. doi: 10.1016/j.jacc.2018.08.2141 [DOI] [PubMed] [Google Scholar]
- 28. Stefanick ML, King AC, Mackey S, Tinker LF, Hlatky MA, LaMonte MJ, Bellettiere J, Larson JC, Anderson G, Kooperberg CL, et al. Women’s health initiative strong and healthy pragmatic physical activity intervention trial for cardiovascular disease prevention: design and baseline characteristics. Journals Gerontol Ser A. 2021;76:725–734. doi: 10.1093/gerona/glaa325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figure S1


