Abstract
Background
Premenopausal women are less likely to develop hypertension and salt‐related complications than are men, yet the impact of sex on mechanisms regulating Na+ homeostasis during dietary salt challenges is poorly defined. Here, we determined whether female rats have a more efficient capacity to acclimate to increased dietary salt intake challenge.
Methods and Results
Age‐matched male and female Sprague Dawley rats maintained on a normal‐salt (NS) diet (0.49% NaCl) were challenged with a 5‐day high‐salt diet (4.0% NaCl). We assessed serum, urinary, skin, and muscle electrolytes; total body water; and kidney Na+ transporters during the NS and high‐salt diet phases. During the 5‐day high‐salt challenge, natriuresis increased more rapidly in females, whereas serum Na+ and body water concentration increased only in males. To determine if females are primed to handle changes in dietary salt, we asked the question whether the renal endothelin‐1 natriuretic system is more active in female rats, compared with males. During the NS diet, female rats had a higher urinary endothelin‐1 excretion rate than males. Moreover, Ingenuity Pathway Analysis of RNA sequencing data identified the enrichment of endothelin signaling pathway transcripts in the inner medulla of kidneys from NS‐fed female rats compared with male counterparts. Notably, in human subjects who consumed an Na+‐controlled diet (3314–3668 mg/day) for 3 days, women had a higher urinary endothelin‐1 excretion rate than men, consistent with our findings in NS‐fed rats.
Conclusions
These results suggest that female sex confers a greater ability to maintain Na+ homeostasis during acclimation to dietary Na+ challenges and indicate that the intrarenal endothelin‐1 natriuretic pathway is enhanced in women.
Keywords: endothelin‐1, natriuresis, nitric oxide, sex differences, sodium
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- ETA
endothelin receptor subtype A
- ETB
endothelin receptor subtype B
- HS
high salt
- IMCD
inner medullary collecting duct
- IPA
Ingenuity Pathway Analysis
- NCC
Na+/Cl− cotransporter
- NHE3
Na+/H+ exchanger isoform 3
- NOx
NO metabolites (nitrite and nitrate)
- NS
normal salt
Clinical Perspective
What Is New?
We report that women have a greater ability to maintain sodium balance during acclimation to dietary sodium challenges.
This study also identifies sex‐specific discrepancies in the renal endothelin system, which plays a critical role in the regulation of sodium excretion and blood pressure.
What Are the Clinical Implications?
This study underscores the importance of studying sex‐specific differences in the mechanisms regulating blood pressure and sodium excretion.
The renal endothelin‐1 system may play an important role on the advanced ability of the female kidney to excrete sodium.
Maintenance of Na+ homeostasis has a fundamental role in blood pressure regulation, and the kidney is crucial in this process. In particular, the kidney tightly regulates natriuresis and has the capacity to acclimate to dietary salt challenges, thus ensuring that fluid‐electrolyte balance is maintained. 1 Although there have been extensive studies over many years, the complex molecular interactions that control Na+ handling in the kidney remain incompletely defined. This has severely complicated the development of therapeutic strategies to prevent salt‐sensitive hypertension.
Despite evidence for male‐female differences in hypertension prevalence and pathophysiology, 2 the vast majority of studies focused on Na+ handling have been restricted to men. Therefore, a clear understanding of sex‐dependent regulation of Na+ homeostasis is critically lacking. Recent studies demonstrated that acute natriuretic responses to an intraperitoneal saline load are more rapid in female than in male rats. 3 Similarly, intravenous infusion of hypertonic saline to humans evoked a more pronounced natriuretic action in women than in men. 4 However, sex discrepancies in mechanistic pathways involved in natriuresis remain poorly characterized.
Of the myriad Na+‐regulatory pathways, the kidney endothelin‐1 signaling system plays an important role in controlling Na+ homeostasis 5 and contributes to sex discrepancies in blood pressure control and fluid and electrolyte homeostasis. 6 , 7 , 8 , 9 , 10 The renal inner medulla contains the highest concentration of endothelin‐1 in the body. 11 Upon release from collecting duct cells, endothelin‐1 inhibits tubular Na+ transport, promoting natriuresis through activation of endothelin receptors and production of NO. 5 Endothelin‐1–induced natriuresis is mediated mainly through activation of endothelin receptor subtype B (ETB) receptors. 5 Interestingly, evidence now indicates a contribution of medullary endothelin receptor subtype A (ETA) receptors in facilitating endothelin‐1–mediated natriuresis in female rats only. 12 However, our previous study revealed that male rats have greater expression of ETA in inner medullary collecting duct (IMCD) cells than females do, whereas no sex difference in ETB receptor expression was detected in these cells. 13
The goal of the current study was 2‐fold. First, we tested the hypothesis that female rats have a more efficient capacity to acclimate to increased dietary salt intake challenge. To address this question, we assessed serum, urinary, skin, and muscle electrolyte levels and the abundance of Na+ transporters in male and female Sprague Dawley rats on a normal‐salt (NS) diet and during the initial several days transitioning to a high‐salt (HS) diet. Second, we determined whether the renal endothelin‐1 natriuretic system is more active in female rats, compared with males. This was achieved by measurement of urinary endothelin‐1 excretion, renal ETA and ETB receptor expression in rats during the NS or HS diet phases. We assessed sex‐specific differential expression of the renal inner medullary transcriptome in NS‐fed rats. To begin determining the clinical relevance of our findings in rats, we assessed sex discrepancies in urinary excretion rates of endothelin‐1 and other Na+‐regulatory factors in healthy humans.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
Male and female age‐matched (13–15 weeks old) Sprague Dawley rats were purchased from Envigo (Indianapolis, IN). Rats were maintained on an NS diet (0.49% NaCl, TD 96208; Envigo) for 2 weeks. Then, a nutrient‐matched HS diet (4% NaCl, TD 92034; Envigo) was introduced for 5 consecutive days. During the entire experimental period, animals were housed in a temperature‐controlled room (22–24 °C) with a 12:12‐hour light‐dark cycle, with free access to water. Experiments were conducted over 4 sequential days to ensure that different stages of the estrus cycle were presented in female rats. All animal protocols were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved in advance by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Clinical Studies
Healthy men and women (age, 33.6±1.8 years; body mass index, 25.8±0.8 kg/m2) consumed controlled diets for 3 days (Na+ intake, 3314–3668 mg/day). Specifically, Na+ intake in all meals, snacks, and drinks was 3314 to 3668 mg/day (3668, 3314, and 3387 mg/day on the first, second, and third day, respectively). On the fourth day and after fasting overnight, the first morning void was discarded. Participants then consumed 4 cups of water. Two hours later, a second urine void was collected to assess urinary Na+, endothelin‐1, NO metabolites (nitrite and nitrate [NOx]), aldosterone, and norepinephrine. Na+ intake in the current human study was based on National Health and Nutrition Examination Survey 2013–2016 data, which reported that Americans consume an average of 3361 mg of sodium per day. 14 Studies involving humans were performed with approval from the University of Alabama at Birmingham Institutional Review Board for Human Use and in accordance with the Declaration of Helsinki. Study participants provided written informed consent before inclusion in the study.
Statistical Analysis
Values are presented as mean±SEM in all figures and Table 1. Values in Table 2 are presented as mean±SD. Statistical tests used for each data set are specified in the figure legend. P<0.05 was considered significant.
Table 1.
Sex Differences in Na+‐Regulatory Factors During Acclimation to an HS Diet Challenge
| Males (n=5–8) | Females (n=6–11) | ANOVA results | |||||
|---|---|---|---|---|---|---|---|
| Urinary excretion rate | NS | Day 1 HS | Day 5 HS | NS | Day 1 HS | Day 5 HS | |
| Endothelin‐1, pg/day per kg | 5.6±0.8 | 10.2±0.5* | 10.6±0.7* | 11.8±3.9 † | 14.6±2.0 | 14.9±2.3 |
P interaction=0.9 P diet=0.2 P sex=0.006 |
| NOx, µmol/day per kg | 22.5±1.0 | 24.6±1.8 | 7.7±0.7* | 20.1±3.5 | 32.7±4.7* | 7.8±0.7* |
P interaction=0.08 P diet<0.0001 P sex=0.5 |
| Aldosterone, µg/day per kg | 14.1±2.3 | 5.8±0.9* | 4.3±0.6* | 17.3±2.9 | 13.3±1.5 | 5.3±0.7* |
P interaction=0.4 P diet<0.0001 P sex=0.18 |
| Norepinephrine, µg/day per kg | 5.2±0.3 | 4.6±0.2 | 4.9±0.5 | 4.0±1.0 | 6.8±1.1 | 5.9±1.1 |
P interaction=0.1 P diet=0.4 P sex=0.4 |
Statistical comparisons were performed by repeated‐measures 2‐way ANOVA with Sidak’s post hoc test for multiple comparisons. HS, high salt; and NS, normal salt.
P<0.05 vs corresponding NS values.
P<0.05 vs corresponding male values.
Table 2.
Characteristics of Human Subjects
| Men (n=9–11) | Women (n=12–14) | P values | |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age, y | 34.9±7.7 | 32.6±10.3 | 0.5 |
| Weight, kg | 85.9±11.2 | 66.1±9.9* | 0.001 |
| Body mass index, kg/m2 | 27.5±2.9 | 24.4±4.1* | 0.04 |
| Urine flow, mL/h per kg | 0.5±0.2 | 0.6±0.3 | 0.2 |
| UNaV, μmol/h per kg | 26.6±22.1 | 33.6±23.6 | 0.7 |
Statistical comparisons were performed by unpaired Student t test. UNaV indicates urinary excretion of Na+.
P<0.05 vs corresponding values in men.
Detailed methodology for all protocols used in this study, including metabolic cage experiments, telemetry, RNA sequencing and pathway analysis, are provided in Data S1. Table S1 lists all primary antibodies used for Western blotting in the present study.
Results
Urinary Electrolyte Excretion
Under steady‐state conditions, no sex differences in water or Na+ intake or in urine electrolyte excretion or flow were observed in animals on an NS diet when normalized to body weight (Figure 1A through 1D, Figure S1A through S1C). Upon challenge with an HS diet, the urinary excretion rate of Na+ increased gradually in males over the 5‐day period, whereas the urinary excretion rate of Na+ in females reached a maximum steady state on day 1 of the HS challenge (Figure 1B). Over the 5‐day HS challenge, males and females reached a positive Na+ balance of 87.2±22.2 and 61.3±9.9 mmol/kg, respectively (P=0.3). Water intake and urine flow increased significantly in female rats (Figure 1C and 1D). In males, increases in urine flow and water intake did not reach statistical significance (Figure 1C and 1D). On day 1 of the HS, the difference between water intake and urine output was 20.3±0.9 and 15.7±2.6 mL/day in males and females, respectively (P=0.1). The urinary excretion rate of K+ did not change in either sex during the HS challenge, whereas the urinary excretion rate of Cl− in both sexes increased in a pattern similar to that of the urinary excretion rate of Na+ (Figure S1B and S1C).
Figure 1. Sex differences in the natriuretic response to a HS diet challenge in rats.
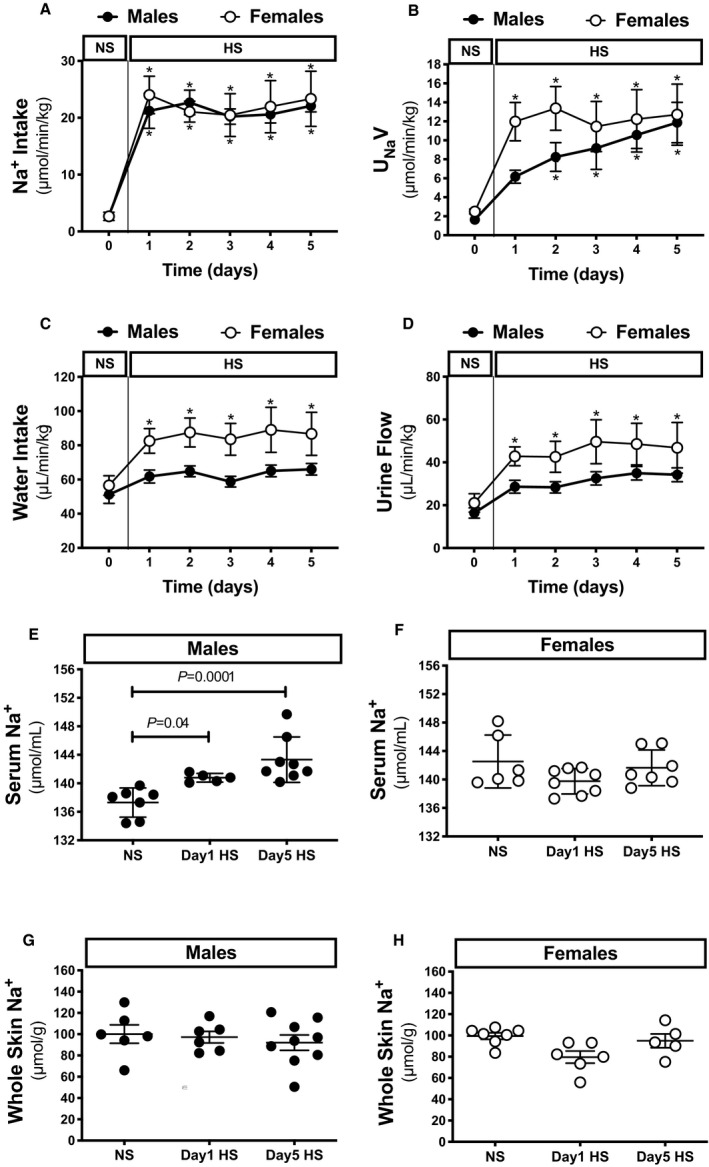
Na+ intake (A), UNaV (B), water intake (C), and urine flow (D) in male and female Sprague Dawley rats during the NS diet phase or the HS challenge. Serum levels of Na+ (E and F) and whole skin Na+ (G and H) relative to dry skin weight in male and female rats during the NS diet phase or on days 1 and 5 of the HS diet challenge (n=5–8 rats in each group). Statistical comparisons were performed by repeated measures 2‐way ANOVA with Sidak’s post hoc test for multiple comparisons (A through D) or two‐way ANOVA with Sidak’s post hoc test for multiple comparisons (E through H). *P<0.05 vs corresponding NS values. ANOVA results: Na+ intake: P interaction=0.9, P time<0.0001, P sex=0.8; UNaV: P interaction=0.5, P time<0.0001, P sex=0.2; water intake: P interaction=0.6, P time=0.002, P sex=0.01; urine flow: P interaction=0.9, P time=0.001, P sex=0.03; serum Na+: P interaction=0.002, P diet=0.02, P sex=0.3; skin Na+: P interaction=0.3, P time=0.2, P sex=0.3. HS indicates high salt; NS, normal salt; and UNaV, urinary excretion of Na+.
Circulating Electrolyte Concentrations
In male rats, the serum Na+ concentration increased on day 1 of the HS challenge and remained high at day 5 (Figure 1E). In contrast, the serum Na+ concentration in female rats did not change during the HS challenge (Figure 1F). Serum K+ concentration increased slightly in both sexes on day 5 of HS (Figure S1D and S1E). Serum Cl− concentration tended to increase during the HS challenge in male rats similar to serum Na+ (Figure S1F and S1G) but did not reach statistical significance (P=0.06 and P=0.07 at day 1 and day 5, respectively; Figure S1F). Serum Cl− concentration did not change in females during the HS challenge.
Blood Pressure
Consistent with previous reports, 2 , 15 mean arterial pressure was lower in female rats than in male rats during the NS diet phase (Figure S2) and mean arterial pressure did not change in either sex during the HS challenge compared with baseline values (Figure S2).
Body Weight and Total Body Water
During the NS phase, males and females weighed 406±3 and 247±3 g, respectively. Body weight did not change in either sex during the HS challenge. However, total body water relative to body weight increased in male rats on day 1 of the HS challenge (from 71.8±0.3% to 72.6±0.4%; P=0.02) and remained elevated on day 5 (72.3±0.4%; P=0.03). In contrast, total body water relative to body weight did not change significantly in females during the HS challenge (NS, 70.6±0.7%; day 1 HS, 71.2±0.8%; day 5 HS, 71.2±0.8%; P=0.3). No sex‐related differences were observed in total body water relative to body weight.
Skin and Muscle Na+
Interestingly, recent findings suggest that skin is also a site of Na+ storage and serves as an extrarenal contributor to the maintenance of Na+ homeostasis and blood pressure regulation as well. 16 , 17 In the present study, skin Na+ concentration in male rats did not change during the HS challenge (Figure 1G). A slight but nonsignificant decline in skin Na+ concentration occurred in female rats on day 1 of the HS challenge (P=0.07; Figure 1H). Neither sex‐ nor diet‐related differences were observed in muscle Na+ concentrations on day 1 of HS (Figure 2A and 2B).
Figure 2. Muscle Na+ and creatinine clearance during the HS diet challenge.
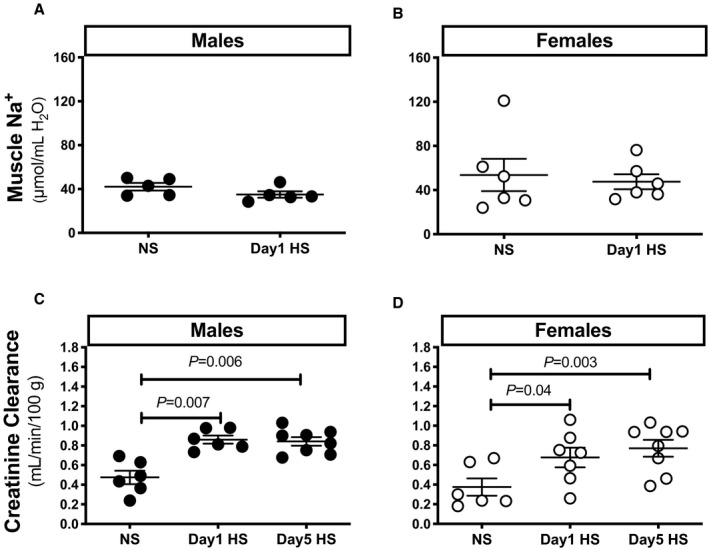
Ratio of muscle Na+ to water (A and B) and creatinine clearance (C and D) in male and female Sprague Dawley rats during the NS diet phase or on day 1 of the HS challenge (n=5–8 rats in each group). Statistical comparisons were performed by 2‐way ANOVA with Sidak’s post hoc test for multiple comparisons. ANOVA results: muscle Na+: P interaction=0.9, P time=0.5, P sex=0.2; creatinine clearance: P interaction=0.7, P time<0.0001, P sex=0.07. HS indicates high salt; and NS, normal salt.
Creatinine Clearance
To evaluate the effects of HS on kidney function, we determined creatinine clearance as an estimate of glomerular filtration rate. Creatinine clearance increased in both sexes on day 1 of the HS challenge, and this increase was maintained at day 5 (Figure 2C and 2D).
Kidney Na+ Transporters
The actions of Na+ transporters along the nephron determine tubular Na+ reabsorption and, consequently, Na+ excretion by the kidney. To assess the potential involvement of these transporters in mediating the sex differences in HS‐induced natriuresis, we determined the protein abundance of Na+/Cl− cotransporter (NCC; total and phosphorylated), Na+/H+ exchanger isoform 3 (NHE3; total and phosphorylated), epithelial Na+ channel α subunit, and Na+, K+‐ATPase α subunit in renal cortices from rats on NS and day 1 of HS (the time at which sex differences in HS‐induced natriuresis were most apparent). The abundance of total NCC and phosphorylated NCC were higher in female rats than in male rats, regardless of diet (Figure 3A and 3B). Total NHE3 was higher in females compared with males on an NS diet. However, neither sex‐ nor diet‐related differences were observed in the ratio of phosphorylated NCC to total NCC, the abundance of phosphorylated NHE3 or Na+, K+‐ATPase α subunit, or the ratio of phosphorylated NHE3 to total NHE3 (Figure 3A through 3E).
Figure 3. Na+ transporter protein expression during the HS diet challenge.
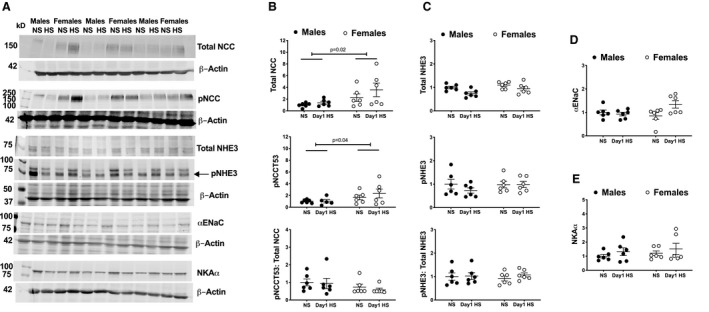
Representative Western blots of Na+ transporters and loading controls are presented (A). Protein abundance of total NCC, pNCC, and the ratio thereof (B), total NHE3 and pNHE3, and the ratio thereof (C), αENaC (D) and NKAα (E), in renal cortices from male and female Sprague Dawley rats during the NS diet phase or on day 1 of the HS challenge (protein abundance is presented relative to corresponding levels in male NS rats (n=6 rats in each group). Statistical comparisons were performed by 2‐way ANOVA with Sidak’s post hoc test for multiple comparisons. ANOVA results: total NCC: P interaction=0.5, P diet=0.2, P sex=0.02; pNCC: P interaction=0.5, P diet=0.2, P sex=0.04; pNCC: total NCC: P interaction=0.8, P diet=0.6, P sex=0.1; total NHE3: P interaction=0.4, P diet=0.01, P sex=0.04; pNHE3: P interaction=0.4, P diet=0.4, P sex=0.4; pNHE3: total NHE3: P interaction=0.6, P diet=0.5, P sex=0.9; αENaC: P interaction=0.04, P diet=0.1, P sex=0.3; NKAα: P interaction=0.9, P diet=0.2, P sex=0.4. αENaC indicates epithelial Na+ channel α subunit HS, high salt; NCC, Na+/Cl− cotransporter; NHE3, Na+/H+ exchanger isoform 3; NKAα, Na+, K+‐ATPase α; NS: normal salt; pNCC, phosphorylated Na+/Cl− cotransporter; and pNHE3, phosphorylated Na+/H+ exchanger isoform 3.
Urinary Excretion of Natriuretic/Antinatriuretic Factors
To assess sex differences in the renal endothelin‐1 pathway during the HS challenge, we measured the 24‐hour excretion rate of endothelin‐1 and NOx) in rats during the NS diet phase and on days 1 and 5 of HS. Among other factors involved in determining the natriuretic response to an HS diet, it is clear that reduced renal sympathetic activity (primarily mediated by norepinephrine) and aldosterone signaling inhibit natriuresis, whereas ANP (atrial natriuretic peptide) promotes natriuresis. Thus, we also measured the 24‐hour excretion rate of aldosterone and norepinephrine and serum ANP during the NS and HS diet phases. Interestingly, the urinary excretion rate of endothelin‐1 was higher in females than in males during the NS diet phase (Table 1). No sex differences were observed in the urinary excretion rate of NOx, aldosterone, or norepinephrine (Table 1) or in the serum ANP concentration under NS diet conditions (males, 0.12±0.04; females, 0.10±0.04 ng/mL).
In males, the urinary excretion rate of endothelin‐1 increased on day 1 of HS, and this change was maintained on day 5 (Table 1). In contrast, the urinary excretion rate of endothelin‐1 did not change in females during the HS challenge (Table 1). The urinary excretion rate of NOx increased in females only on day 1 of HS. In both sexes, however, this rate decreased on day 5 of HS, such that it was significantly lower than the rate during the NS diet phase (Table 1). The urinary excretion rate of aldosterone decreased in males on day 1 of the HS challenge, and this decrease was maintained on day 5 (Table 1). However, the urinary excretion rate of aldosterone decreased in females only on day 5 of the HS challenge (Table 1). Neither the urinary excretion rate of norepinephrine (Table 1) nor the serum ANP concentration changed on day 1 of the HS challenge in either sex (males, 0.14±0.04; females, 0.11±0.03 ng/mL).
RNA Sequencing and Ingenuity Pathway Analysis
Inner medullae, which have an important role in the fine‐tuning of urinary Na+ excretion, were collected from NS‐fed rats and subjected to RNA sequencing. Raw data files, a read summary with mapping rate, and a summary file of transcripts and statistical results from RNA sequencing have been deposited in the Gene Expression Omnibus database under the accession number GSE136387. A list of transcripts that met the following criteria were uploaded to Ingenuity Pathway Analysis (IPA; Qiagen) for core analysis: (1) detected in both male and female samples, and (2) identified to be differentially expressed by Cufflinks or at least a 1.5‐fold different between the sexes. Transcripts meeting these criteria are represented by color‐coded points in Figure S3. Of the 16 061 annotated transcripts detected in the RNA sequencing analysis, 3.43% (551) were exclusively expressed in females, 11.10% (1782) were expressed in both sexes but significantly higher ‘and’ or ‘or’ 1.5‐fold higher in females, 5.32% (855) were expressed in both sexes but significantly higher ‘and’ or ‘or’ 1.5‐fold higher in males, 2.37% (381) were exclusively expressed in males, and 77.78% (12 492) appeared to be similarly expressed in both sexes (Figure 4A).
Figure 4. Sex differences in endothelin‐1 signaling pathway activation in the inner medulla under NS conditions.
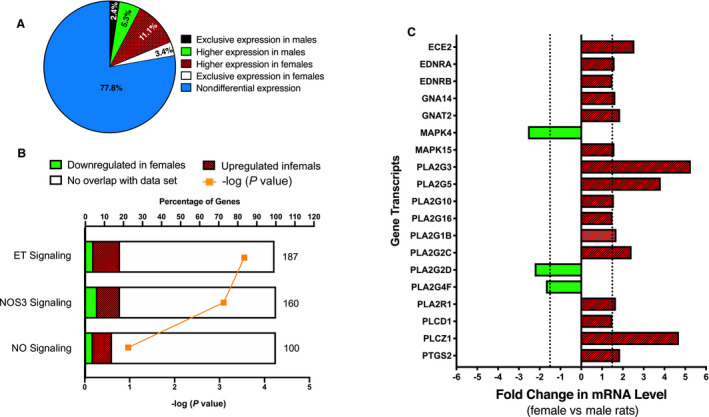
Inner medullae were collected from NS‐fed Sprague Dawley rats (n=8/sex) and pooled (n=4 rats/pool/group) by sex for RNA sequencing analysis and subsequent IPA. Graphical representation of the sex differences in the renal inner medullary transcriptome (A). Graphical representation of the sex differences in transcript expression found within enriched IPA pathways with established roles in natriuresis (ET signaling, NOS3 signaling, and NO signaling in the cardiovascular system) (B). Pathways are ranked by –log (P value). The total number of genes found within each pathway is shown on the right of the respective bar. Graphical represenation of the relative abundance of inner medullary gene transcripts involved in the endothelin‐1 natriuretic signaling pathway in male and female rats (C). Gene transcripts that were differentially expressed and/or altered by at least 1.5‐fold in female rats relative to males are shown. IPA indicates Ingenuity Pathway Analysis; NOS, nitric oxide synthase; and NS, normal salt.
The signaling pathways with the most differentially expressed genes were ranked by −log (P value) in the IPA software and are reported in Table S2. The top 10 highly differentially expressed genes between sexes in the renal inner medullary transcriptome are listed in Table S3. Figure 4B presents changes in transcripts found within enriched IPA pathways that have an established role in natriuretic function, including “endothelin signaling,” “NO synthase 3 signaling,” and “NO signaling in the cardiovascular system.” These pathways were predicted to be activated (positive z score) in females relative to males (Figure 4B). Other pathways that were enriched in females relative to males include G‐protein coupled receptor signaling; cAMP‐mediated signaling; calcium signaling; phospholipases; and interleukin‐8, ‐9, and ‐10 signaling (Table S2). IPA identified an enrichment of transcripts of the endothelin‐1 signaling pathway in females relative to males (P=0.0003), with 34 of 187 pathway transcripts being differentially expressed or with at least a 1.5‐fold change in abundance between sexes (Figure 4B).
Renal Endothelin‐1
Of the 34 transcripts in the endothelin‐1 signaling pathway that were differentially expressed in a sex‐dependent manner, 19 transcripts were experimentally determined to be involved in the endothelin‐1 production/signaling cascade in IMCD cells 5 (Figure 4C). As shown, 16 of the 19 (84.2%) transcripts involved in the endothelin‐1 natriuretic pathway in IMCD cells were enriched in inner medullae from females compared with those of males (Figure 4C). Of note, inner medulla tissue contains the highest concentration of endothelin‐1 and ETB in the body. 11
Endothelin‐1 evokes natriuresis primarily via activation of the ETA and ETB receptors. 8 , 12 , 18 Thus, we measured ETA and ETB receptor expression in the cortex and inner and outer medulla of the kidneys from rats on the NS diet or day 1 of the HS challenge. Neither ETA nor ETB mRNA expression changed significantly in the renal cortical (Figure 5A through 5D) or inner medullary (Figure 5I through 5L) tissues of either sex upon HS challenge. However, ETB receptor expression increased within the outer medullae in males (Figure 5G), whereas ETA receptor expression increased within the outer medullae of females upon HS challenge (Figure 5F).
Figure 5. HS diet challenge increases renal outer medullary endothelin receptor expression in a sex‐dependent manner.
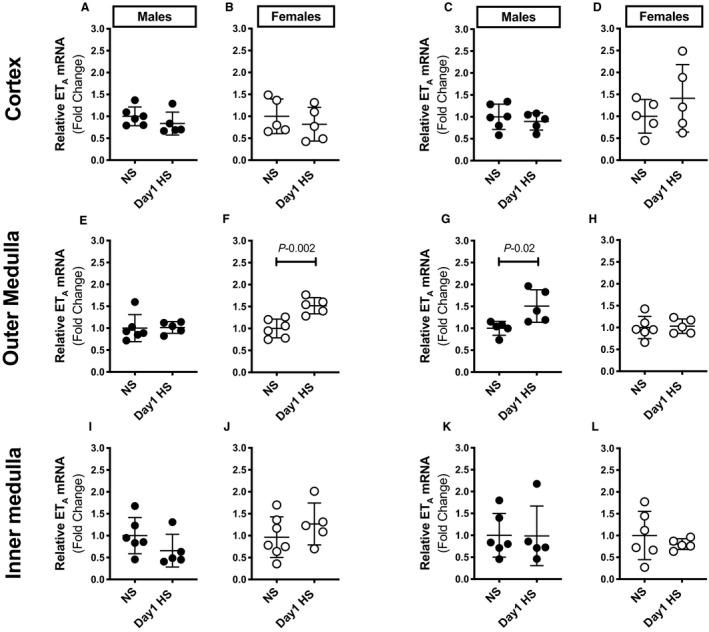
mRNA expression of cortical (A through D), outer medullary (E through H), and inner medullary (I through L) ETA and ETB receptors in male and female Sprague Dawley rats during the NS diet phase or on day 1 of the HS diet challenge (n=5–6 rats in each group). Gene expression values represent the fold change from corresponding NS levels. Statistical comparisons were performed by unpaired Student t test. P<0.05 vs corresponding NS values. ETA indicates endothelin receptor subtype A; ETB, endothelin receptor subtype B; HS, high salt; and NS, normal salt.
Kidney Interleukin‐1β
Given that interleukin‐1β stimulates renal endothelin‐1 production in vivo and in vitro, 19 , 20 , 21 we also determined sex and dietary effects on renal interleukin‐1β expression. Immunohistochemical analysis of tissues obtained from rats during the NS diet phase showed the expression of interleukin‐1β tended to be elevated in the renal cortex of females relative to that of males (Figure 6A), although interleukin‐1β localization in the kidney was similar in both sexes. Within the cortex of NS‐fed rats, mesangial cells in the glomeruli and cortical brush border stained positive for interleukin‐1β (Figure 6A). ELISA measurements revealed that renal cortical interleukin‐1β expression significantly increased on day 1 of the HS challenge in males but not in females (Figure 6B). No diet‐induced differences in interleukin‐1β expression were observed in the inner medulla, when compared with corresponding NS values (Figure 6C). A sex difference in inner medullary interleukin‐1β expression was observed (Figure 6C). However, male rats had exaggerated cortical interleukin‐1β expression at the brush border and generalized staining in the tubular cell cytoplasm on day 1 of the HS challenge. In the females, tubular staining of interleukin‐1β at day 1 was limited to the brush border, at levels similar to those observed on the NS diet, and punctate staining within the cortical proximal tubules.
Figure 6. Renal cortical interleukin‐1β expression during the HS diet challenge.
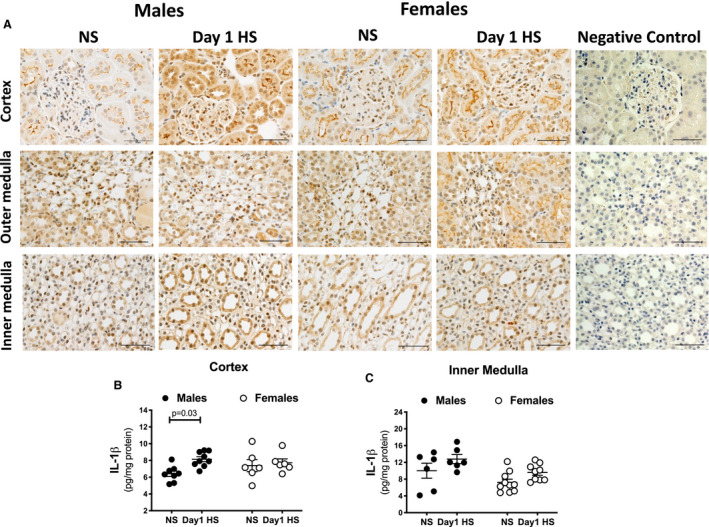
Representative images of IL‐1β protein expression in the renal cortex and outer and inner medulla of male and female Sprague Dawley rats during the NS diet phase or on day 1 of the HS challenge (n=5–6 rats in each group; scale bar, 20 μm). Negative control images are represented (A). Protein levels of IL‐1β measured by ELISA in renal cortex (B) and inner medulla (C) of male and female Sprague Dawley rats during the NS diet phase or on day 1 of the HS challenge (n=6–10 rats in each group). Brown color corresponds to IL‐1β‐positive staining. Statistical comparisons were performed by two‐way ANOVA with Sidak’s post hoc test for multiple comparisons. ANOVA results: cortex: P interaction=0.1, P diet=0.03, P sex=0.5; inner medulla: P interaction=0.8, P diet=0.02, P sex=0.008. HS indicates high salt; IL, interleukin; and NS, normal salt.
Na+ Regulatory Factors in Humans
To assess the relevance of our findings in humans, we measured the urinary excretion rate of endothelin‐1, NOx, aldosterone, and norepinephrine in urine specimens collected from subjects maintained on an Na+‐controlled diet. Subject characteristics are provided in Table 2. Of note, women excreted significantly more endothelin‐1 than men (Figure 7A). No sex differences were observed in the urinary excretion of NOx, aldosterone, or norepinephrine (Figure 7B through 7D).
Figure 7. Women have higher urinary endothelin‐1 levels than men.
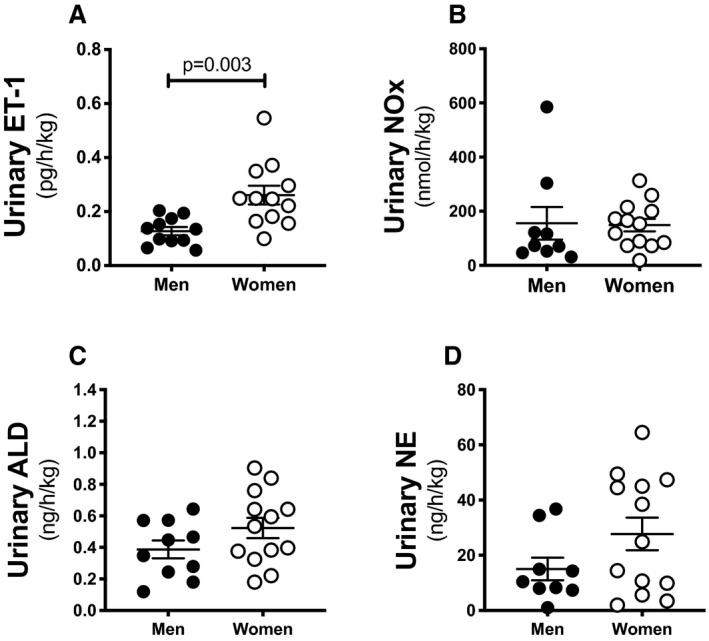
Urinary excretion of endothelin‐1 (A) nitrite/nitrate (NOx) (B), ALD (C), and NE (D) in healthy men and women (n=9–13 subjects in each group). Statistical comparison was performed by unpaired Student t test. ET‐1 indicates endothelin‐1; ALD indicates aldosterone; and NE, norepinephrine.
Discussion
A central dogma in kidney physiology is that it takes the body ≈3 days to reach steady‐state urinary Na+ excretion, where Na+ intake matches excretion. 1 Consistent with this idea, we observed that urinary Na+ excretion in male rats increases gradually in response to the induction of an HS diet, reaching a new steady state in 3 to 5 days. In striking contrast, female rats elicited robust increases in urinary Na+ excretion on day 1 of HS. This observation indicates that females are primed to handle salt challenges more efficiently than males, which is in line with recent data. 3 Similar to our findings in rats, a previous clinical study found that the magnitude of the increase in urinary Na+ excretion during hypertonic saline infusion is lower in men than in women. 4
In the current rat study, HS‐induced water intake and urine output was more pronounced in females. Dickinson et al 22 reported similar results in spiny mice fed an HS diet for 7 days. Supporting these results, sex and sex hormones have been shown to regulate osmoreceptors that control thirst. 4 , 23 , 24 , 25 , 26 It has also been shown that estrogen supplementation to ovariectomized rats increases water intake and urine flow. 27 Notably, pretreatment with angiotensin II decreased water intake in male but not female rats. 28 Central interactions between estrogen and angiotensin II control the drinking behavior in rats. 29 , 30 The specific mechanism underlying the sex difference in water intake in the present studies remains to be determined, but sex hormonal–induced differences in the dipsogenic potential of angiotensin II is a reasonable target for future studies.
The HS‐induced augmentation in total body water observed only in male rats may appear paradoxical with respect to their relatively lower water intake. However, the difference between water intake and urine output being ≈4.6 mL higher in males versus females on day 1 of the HS would provide a proper explanation for the observed ≈1% increase in total body water relative to body weight in males (body weight average, 406 g). Consistent with our findings, previous work showed that total body water does not increase in female rats in response to 1% NaCl in drinking water. 31
Our observation that serum Na+ concentration in female rats remained unchanged while males had an increase during acclimation to HS suggests that females have a greater capacity for maintaining circulating levels of Na+. Of note, postprandial increases in circulating levels of Na+ impair NO‐dependent vasodilation in healthy subjects. 32 The elevation in circulating Na+ that we observed in males only suggests that males may be more susceptible to salt‐related complications during acclimation to salt challenges. Earlier reports demonstrated that plasma obtained from salt‐loaded subjects inhibited erythrocyte Na+/K+/Cl− cotransport, 33 which may contribute to alterations in plasma electrolytes.
We did not observe any effect on Na+ transporter expression in the kidney cortex on day 1 of HS. However, the abundance of total and phosphorylated NCC was higher in the kidneys of females than in those of males, which is consistent with recently published findings. 3 Assuming that abundance is proportional to activity, these sex differences appear to be opposite to what would be presumed. However, control of transporter activity is complex and has many levels of regulation. Additional studies are warranted to determine whether sex differences in the regional distribution of Na+ transporters 3 and transporter trafficking may contribute to acclimation to HS. Recently, Tahaei and colleagues 34 revealed that the distal convoluted tubule of female mice expresses a greater density of NCC in a shorter distal convoluted tubule structure. Interestingly, the female distal convoluted tubule has greater structural remodeling capacity to elongate in response to loop diuretics. 34 Altogether, it is clear that the female kidney is equipped with unique mechanisms to adapt to unique physiological challenges.
Upon HS challenge, the rate of urinary endothelin‐1 excretion increased in male rats only, consistent with previous reports of HS diets increasing renal endothelin‐1 production and activity. 35 , 36 , 37 Our RNA sequencing data demonstrated enrichment in the endothelin‐1 signaling pathway in female rats. In addition, the rate of urinary endothelin‐1 excretion was higher in female rats than in males. Our study does not conclusively demonstrate that kidney endothelin‐1 signaling is the primary cause for the sex differences in HS‐induced natriuresis but does show that the kidney endothelin‐1 system is highly activated under basal conditions in females. The differential HS‐induced overexpression of outer medullary ETB in males and ETA in females is interesting and suggests a female‐specific contribution of ETA in the natriuretic response to salt, which aligns with previous studies. 8 Furthermore, ETA and ETB can work cooperatively to facilitate Na+ and water excretion. 8 , 36 , 38 , 39 Future studies are required to determine the effect of salt on renal ET receptor protein abundance and localization.
Since the NO pathway is known to contribute to endothelin‐1–induced natriuresis, we also measured the excretion of urinary NOx as a measure of NO production. The increase in urinary NOx excretion in female rats on day 1 of HS may provide an explanation for the more rapid HS‐induced natriuresis observed in females. IPA also revealed an enrichment of NO and nitric oxide synthase 3 signaling pathways in inner medullae from female rats. Importantly, it has been shown that high levels of urinary NOx occur upon collecting duct endothelin‐1 production. 37 This may suggest that endothelin‐1 and NO signaling pathways work cooperatively to prime efficient natriuresis in females.
Control of natriuresis is complex and involves numerous signaling pathways in several systems. We postulate that sex steroids, possibly ovarian hormones, facilitate HS‐induced natriuresis. Indeed, salt sensitivity is reported to be increased after menopause. 40 Ovariectomy has been shown to exacerbate salt sensitivity in Dahl salt‐sensitive rats. 41 This is relevant to our recent study demonstrating a female‐specific natriuretic response to activation of G protein–coupled estrogen receptors. 38 , 42 Therefore, it is possible, and perhaps likely, that additional pathways are involved in the enhanced efficiency of females to handle dietary Na+ challenges. Although we did not detect sex‐specific differences in aldosterone, norepinephrine, or NO excretion when rats were maintained on a NS diet, the contribution of sex differences in the downstream signaling cascades for these Na+ regulatory factors in sex differences in acclimation to HS remains to be determined. Earlier studies have reported changes in urinary excretion of aldosterone and norepinephrine during the estrus cycle in sheep and rats, respectively. 43 , 44 , 45 In addition, ETA and ETB mRNA expression and nitric oxide synthase activity in female rat kidneys are regulated by estradiol. 46 , 47 Accordingly, we anticipate that the increased variability in the urinary excretion levels of Na+‐regulatory factors in female rats would be related to different phases of the estrus cycle.
The inflammatory cytokine interleukin‐1β has been shown to provoke endothelin‐1 production in various cell types, including kidney epithelial cells, 20 and treatment of mouse IMCD‐3 cells with interleukin‐1β has been shown to promote endothelin‐1 release, at least partially, via activation of NF‐κB (nuclear factor kappa‐light‐chain‐enhancer of activated B cells). 21 Furthermore, Boesen et al 21 demonstrated that systemic infusion of interleukin‐1β promotes urinary endothelin‐1 excretion in male rats. We did not observe a greater interleukin‐1β expression in the female kidney of NS‐fed rats, suggesting that the interleukin‐1β/endothelin‐1 axis may not be as important in females as in males. This may be related to potential sex differences in other factors that regulate endothelin‐1 such as the transcription factor tonicity‐responsive enhancer‐binding protein. 48 , 49 The increase in cortical interleukin‐1β on day 1 of the HS in male rats is well poised to mediate the male‐specific increase in urinary endothelin‐1 excretion observed at this time point.
Data from human studies have indicated a significant correlation between urinary endothelin‐1, NOx, and natriuresis. 50 Further studies suggested that impairments in the kidney endothelin‐1 system may contribute to the development of essential hypertension and salt sensitivity. 51 , 52 , 53 Interestingly, it has been shown that patients with essential hypertension have reduced urinary endothelin‐1 excretion. 52 An early study revealed that salt‐sensitive men and women have lower urinary endothelin‐1 excretion than their salt‐resistant counterparts. 52 Despite extensive evidence of sex differences in the kidney endothelin‐1 pathway in animal studies, little is known about the role of kidney endothelin‐1 in regulating renal Na+ handling in men and women. Correlating with results from our animal studies, we discovered that women excrete more endothelin‐1 than men. This is consistent with the hypothesis that women are primed to better handle acute challenges to dietary sodium intake.
Conclusions
In conclusion, there are clear sex differences in the mechanisms involved in maintaining fluid and electrolyte homeostasis, and women have an efficient capacity to manage dietary Na+ challenges. Defining of these mechanistic pathways may lead to the development of sex‐specific therapies for salt‐sensitive hypertension and other salt‐related health complications. Given that an HS diet is one of the major risk factors for the development of hypertension, the enhanced capacity of women to handle salt challenges may contribute to the female protection against hypertension during their premenopausal age, compared with postmenopausal women.
Sources of Funding
This project was supported by R00DK119413, K99DK119413, American Heart Association 18CDA34110010 and 15POST25090329 to Dr Gohar; K01HL145324 to Dr De Miguel; T32HL007918‐19 and American Physiological Society Porter Fellowship to Dr Obi; R25 DK115353 Kidney Undergraduate Research Experience to Daugherty; F31 DK111067 to Dr Sedaka; American Heart Association 19POST34380109 to Dr Becker; K01DK105038 and R03DK120503 to Dr Hyndman, American Heart Association 15PRE25560074 and T32 GM008111 to Dr Johnston, as well as P30DK079337 to the University of Alabama at Birmingham/University of California at San Diego O’Brien Core Research Center; P30DK056336, P30DK079626 and PAG050886A to the University of Alabama at Birmingham Small Animal Phenotyping Core; K01DK106284 to Dr Mitchell; and P01 HL 069999, P01 HL136267, and American Heart Association SFRN 15SFRN24420002 and SRG 19SRG34930023 to Drs Pollock and Pollock.
Disclosures
Dr Gohar is also affiliated with the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Egypt. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S3
Figures S1–S3
References 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71
Acknowledgments
We appreciate the assistance of Drs Celso Gomez‐Sanchez and Elise Gomez‐Sanchez with aldosterone assays. We also gratefully acknowledge the excellent technical assistance of Dr Xiaofen Liu with the immunohistochemistry studies, Rawan Almutlaq with ANP assays, and John Miller Allan with atomic absorption measurements. We acknowledge support from the University of Alabama at Birmingham /UCSD O’Brien Core Center for Acute Kidney Injury Research for creatinine measurements, the University of Alabama at Birmingham Small Animal Phenotyping Core for the quantitative magnetic resonance measurements, and the Genomic Sciences and Precision Medicine Center Sequencing Core at the Medical College of Wisconsin. The authors would like to extend their sincere gratitude to the late Dr Ijeoma Obi for her hard work and dedication to this project.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Saunders Elsevier; 2019. [Google Scholar]
- 2. Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran AN, Castelo‐Branco RC, Pastor‐Soler N, Arranz CT, Yu ASL, et al. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol. 2017;28:3504–3517. doi: 10.1681/ASN.2017030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol (1985). 2001;91:1893–1901. [DOI] [PubMed] [Google Scholar]
- 5. Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol. 2011;1:883–919. doi: 10.1002/cphy.c100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci (Lond). 2008;114:85–97. doi: 10.1042/CS20070169 [DOI] [PubMed] [Google Scholar]
- 7. Kittikulsuth W, Sullivan JC, Pollock DM. ET‐1 actions in the kidney: evidence for sex differences. Br J Pharmacol. 2013;168:318–326. doi: 10.1111/j.1476-5381.2012.01922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1‐dependent natriuresis in female rats. Hypertension. 2009;53:324–330. doi: 10.1161/HYPERTENSIONAHA.108.123687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gohar EY, Pollock DM. Sex‐specific contributions of endothelin to hypertension. Curr Hypertens Rep. 2018;20:58. doi: 10.1007/s11906-018-0856-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gohar EY, Giachini FR, Pollock DM, Tostes RC. Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases. Life Sci. 2016;159:20–29. doi: 10.1016/j.lfs.2016.02.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitamura K, Tanaka T, Kato J, Eto T, Tanaka K. Regional distribution of immunoreactive endothelin in porcine tissue: abundance in inner medulla of kidney. Biochem Biophys Res Commun. 1989;161:348–352. doi: 10.1016/0006-291X(89)91603-3 [DOI] [PubMed] [Google Scholar]
- 12. Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension. 2011;58:212–218. doi: 10.1161/HYPERTENSIONAHA.111.172734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin C, Speed JS, Hyndman KA, O'Connor PM, Pollock DM. Sex differences in ET‐1 receptor expression and Ca2+ signaling in the IMCD. Am J Physiol Renal Physiol. 2013;305:F1099–F1104. doi: 10.1152/ajprenal.00400.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wallace TC, Cowan AE, Bailey RL. Current sodium intakes in the United States and the modelling of glutamate’s incorporation into select savory products. Nutrients. 2019;11:2691. doi: 10.3390/nu11112691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu W, Sui D, Ye H, Ouyang Z, Wei Y. CYP2C11 played a significant role in down‐regulating rat blood pressure under the challenge of a high‐salt diet. PeerJ. 2019;7:e6807. doi: 10.7717/peerj.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–F1117. doi: 10.1152/ajprenal.00200.2003 [DOI] [PubMed] [Google Scholar]
- 17. Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A, Luft FC. Spooky sodium balance. Kidney Int. 2014;85:759–767. doi: 10.1038/ki.2013.367 [DOI] [PubMed] [Google Scholar]
- 18. Stuart D, Chapman M, Rees S, Woodward S, Kohan DE. Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346:182–189. doi: 10.1124/jpet.113.205286 [DOI] [PubMed] [Google Scholar]
- 19. Corder R, Carrier M, Khan N, Klemm P, Vane JR. Cytokine regulation of endothelin‐1 release from bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1995;26(suppl 3):S56–S58. doi: 10.1097/00005344-199506263-00018 [DOI] [PubMed] [Google Scholar]
- 20. Ohta K, Hirata Y, Imai T, Kanno K, Emori T, Shichiri M, Marumo F. Cytokine‐induced release of endothelin‐1 from porcine renal epithelial cell line. Biochem Biophys Res Commun. 1990;169:578–584. doi: 10.1016/0006-291X(90)90370-3 [DOI] [PubMed] [Google Scholar]
- 21. Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin‐1beta, but not interleukin‐6, enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol. 2008;295:F446–F453. doi: 10.1152/ajprenal.00095.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickinson H, Moritz KM, Kett MM. A comparative study of renal function in male and female spiny mice—sex specific responses to a high salt challenge. Biol Sex Differ. 2013;4:21. doi: 10.1186/2042-6410-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spruce BA, Baylis PH, Burd J, Watson MJ. Variation in osmoregulation of arginine vasopressin during the human menstrual cycle. Clin Endocrinol (Oxf). 1985;22:37–42. doi: 10.1111/j.1365-2265.1985.tb01062.x [DOI] [PubMed] [Google Scholar]
- 24. Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL. Osmoregulation of thirst and vasopressin during normal menstrual cycle. Am J Physiol. 1988;254:R641–R647. doi: 10.1152/ajpregu.1988.254.4.R641 [DOI] [PubMed] [Google Scholar]
- 25. Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. 2002;283:E711–E721. doi: 10.1152/ajpendo.00192.2002 [DOI] [PubMed] [Google Scholar]
- 26. Perinpam M, Ware EB, Smith JA, Turner ST, Kardia SL, Lieske JC. Key influence of sex on urine volume and osmolality. Biol Sex Differ. 2016;7:12. doi: 10.1186/s13293-016-0063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheema MU, Irsik DL, Wang Y, Miller‐Little W, Hyndman KA, Marks ES, Frokiaer J, Boesen EI, Norregaard R. Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor alpha. Am J Physiol Renal Physiol. 2015;309:F305–F317. doi: 10.1152/ajprenal.00685.2014 [DOI] [PubMed] [Google Scholar]
- 28. Santollo J, Volcko KL, Daniels D. Sex differences in the behavioral desensitization of water intake observed after repeated central injections of angiotensin II. Endocrinology. 2018;159:676–684. doi: 10.1210/en.2017-00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jonklaas J, Buggy J. Angiotensin‐estrogen central interaction: localization and mechanism. Brain Res. 1985;326:239–249. doi: 10.1016/0006-8993(85)90033-2 [DOI] [PubMed] [Google Scholar]
- 30. Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II‐induced water intake in the rat. Am J Physiol. 1999;276:R90–R96. doi: 10.1152/ajpregu.1999.276.1.R90 [DOI] [PubMed] [Google Scholar]
- 31. Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Eckardt KU, Hilgers KF. Internal sodium balance in DOCA‐salt rats: a body composition study. Am J Physiol Renal Physiol. 2005;289:F793–F802. doi: 10.1152/ajprenal.00096.2005 [DOI] [PubMed] [Google Scholar]
- 32. Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high‐salt meal in healthy subjects. Am J Clin Nutr. 2011;93:500–505. doi: 10.3945/ajcn.110.006155 [DOI] [PubMed] [Google Scholar]
- 33. Pares I, de la Sierra A, Coca A, Lluch MM, Urbano‐Marquez A, Garay R. Detection of a circulating inhibitor of the Na(+)‐K(+)‐Cl(‐) cotransport system in plasma and urine after high salt intake. Am J Hypertens. 1995;8:965–969. doi: 10.1016/0895-7061(95)00215-4 [DOI] [PubMed] [Google Scholar]
- 34. Tahaei E, Coleman R, Saritas T, Ellison DH, Welling PA. Distal convoluted tubule sexual dimorphism revealed by advanced 3D imaging. Am J Physiol Renal Physiol. 2020;319:F754–F764. doi: 10.1152/ajprenal.00441.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol. 2007;292:F185–F191. doi: 10.1152/ajprenal.00021.2006 [DOI] [PubMed] [Google Scholar]
- 36. Boesen EI, Pollock DM. Cooperative role of ETA and ETB receptors in mediating the diuretic response to intramedullary hyperosmotic NaCl infusion. Am J Physiol Renal Physiol. 2010;299:F1424–F1432. doi: 10.1152/ajprenal.00015.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hyndman KA, Dugas C, Arguello AM, Goodchild TT, Buckley KM, Burch M, Yanagisawa M, Pollock JS. High salt induces autocrine actions of ET‐1 on inner medullary collecting duct NO production via upregulated ETB receptor expression. Am J Physiol Regul Integr Comp Physiol. 2016;311:R263–R271. doi: 10.1152/ajpregu.00016.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gohar EY, Pollock DM. Functional interaction of endothelin receptors in mediating natriuresis evoked by G protein–coupled estrogen receptor 1. J Pharmacol Exp Ther. 2021;376:98–105. doi: 10.1124/jpet.120.000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2008;295:F1635–F1640. doi: 10.1152/ajprenal.90279.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim JM, Kim TH, Lee HH, Lee SH, Wang T. Postmenopausal hypertension and sodium sensitivity. J Menopausal Med. 2014;20:1–6. doi: 10.6118/jmm.2014.20.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sartori‐Valinotti JC, Venegas‐Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt‐sensitive rats. Steroids. 2010;75:794–799. doi: 10.1016/j.steroids.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gohar EY, Daugherty EM, Aceves JO, Sedaka R, Obi IE, Allan JM, Soliman RH, Jin C, De Miguel C, Lindsey SH, et al. Evidence for G‐protein‐coupled estrogen receptor as a pronatriuretic factor. J Am Heart Assoc. 2020;9:e015110. doi: 10.1161/JAHA.119.015110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michell AR. Water and electrolyte excretion during the oestrous cycle in sheep. Q J Exp Physiol Cogn Med Sci. 1979;64:79–88. doi: 10.1113/expphysiol.1979.sp002466 [DOI] [PubMed] [Google Scholar]
- 44. Ozaki Y, Wurtman RJ, Alonso R, Lynch HJ. Melatonin secretion decreases during the proestrous stage of the rat estrous cycle. Proc Natl Acad Sci USA. 1978;75:531–534. doi: 10.1073/pnas.75.1.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wurtman RJ, Ozaki Y. Physiological control of melatonin synthesis and secretion: mechanisms, generating rhythms in melatonin, methoxytryptophol, and arginine vasotocin levels and effects on the pineal of endogenous catecholamines, the estrous cycle, and environmental lighting. J Neural Transm Suppl. 1978:59–70. [PubMed] [Google Scholar]
- 46. Gohar EY, Yusuf C, Pollock DM. Ovarian hormones modulate endothelin A and B receptor expression. Life Sci. 2016;159:148–152. doi: 10.1016/j.lfs.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santmyire BR, Venkat V, Beinder E, Baylis C. Impact of the estrus cycle and reduction in estrogen levels with aromatase inhibition, on renal function and nitric oxide activity in female rats. Steroids. 2010;75:1011–1015. doi: 10.1016/j.steroids.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lakshmipathi J, Wheatley W, Kumar A, Mercenne G, Rodan AR, Kohan DE. Identification of NFAT5 as a transcriptional regulator of the EDN1 gene in collecting duct. Am J Physiol Renal Physiol. 2019;316:F481–F487. doi: 10.1152/ajprenal.00509.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pandit MM, Gao Y, van Hoek A, Kohan DE. Osmolar regulation of endothelin‐1 production by the inner medullary collecting duct. Life Sci. 2016;159:135–139. doi: 10.1016/j.lfs.2015.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jackson RW, Treiber FA, Harshfield GA, Waller JL, Pollock JS, Pollock DM. Urinary excretion of vasoactive factors are correlated to sodium excretion. Am J Hypertens. 2001;14:1003–1006. doi: 10.1016/S0895-7061(01)02169-0 [DOI] [PubMed] [Google Scholar]
- 51. Hwang YS, Hsieh TJ, Lee YJ, Tsai JH. Circadian rhythm of urinary endothelin‐1 excretion in mild hypertensive patients. Am J Hypertens. 1998;11:1344–1351. doi: 10.1016/S0895-7061(98)00170-8 [DOI] [PubMed] [Google Scholar]
- 52. Hoffman A, Grossman E, Goldstein DS, Gill JR Jr, Keiser HR. Urinary excretion rate of endothelin‐1 in patients with essential hypertension and salt sensitivity. Kidney Int. 1994;45:556–560. doi: 10.1038/ki.1994.72 [DOI] [PubMed] [Google Scholar]
- 53. Zoccali C, Leonardis D, Parlongo S, Mallamaci F, Postorino M. Urinary and plasma endothelin 1 in essential hypertension and in hypertension secondary to renoparenchymal disease. Nephrol Dial Transplant. 1995;10:1320–1323. [PubMed] [Google Scholar]
- 54. Jin C, Jeon Y, Kleven DT, Pollock JS, White JJ, Pollock DM. Combined endothelin a blockade and chlorthalidone treatment in a rat model of metabolic syndrome. J Pharmacol Exp Ther. 2014;351:467–473. doi: 10.1124/jpet.114.215566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson MS, Smith DL Jr, Nagy TR. Validation of quantitative magnetic resonance (QMR) for determination of body composition in rats. Int J Body Compos Res. 2009;7:99–107. [PMC free article] [PubMed] [Google Scholar]
- 56. Speed JS, Heimlich JB, Hyndman KA, Fox BM, Patel V, Yanagisawa M, Pollock JS, Titze JM, Pollock DM. Endothelin‐1 as a master regulator of whole‐body Na+ homeostasis. FASEB J. 2015;29:4937–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Young S, Struys E, Wood T. Quantification of creatine and guanidinoacetate using GC‐MS and LC‐MS/MS for the detection of cerebral creatine deficiency syndromes. Curr Protoc Hum Genet. 2007;Chapter 17:Unit 17.3. doi: 10.1002/0471142905.hg1703s54 [DOI] [PubMed] [Google Scholar]
- 58. Manolopoulou J, Bielohuby M, Caton SJ, Gomez‐Sanchez CE, Renner‐Mueller I, Wolf E, Lichtenauer UD, Beuschlein F, Hoeflich A, Bidlingmaier M. A highly sensitive immunofluorometric assay for the measurement of aldosterone in small sample volumes: validation in mouse serum. J Endocrinol. 2008;196:215–224. doi: 10.1677/JOE-07-0134 [DOI] [PubMed] [Google Scholar]
- 59. Foster JM, Carmines PK, Pollock JS. PP2B‐dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol. 2009;297:F471–F480. doi: 10.1152/ajprenal.90760.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid‐electrolyte homeostasis and blood pressure. Hypertension. 2013;62:91–98. doi: 10.1161/HYPERTENSIONAHA.113.01291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA‐seq reads. Nat Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA‐Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Miguel C, Obi IE, Ho DH, Loria AS, Pollock JS. Early life stress induces immune priming in kidneys of adult male rats. Am J Physiol Renal Physiol. 2018;314:F343–F355. doi: 10.1152/ajprenal.00590.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hyndman KA, Mironova EV, Giani JF, Dugas C, Collins J, McDonough AA, Stockand JD, Pollock JS. Collecting duct nitric oxide synthase 1β activation maintains sodium homeostasis during high sodium intake through suppression of aldosterone and renal angiotensin II pathways. J Am Heart Assoc. 2017;6:e006896. doi: 10.1161/JAHA.117.006896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)‐ATPase alpha‐subunit in Drosophila melanogaster . EMBO J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II‐dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol. 2013;305:F510–F519. doi: 10.1152/ajprenal.00183.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing‐Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14 [DOI] [PubMed] [Google Scholar]
- 69. Yang L, Leong PK, Chen JO, Patel N, Hamm‐Alvarez SF, McDonough AA. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol. 2002;282:F730–F740. doi: 10.1152/ajprenal.00298.2001 [DOI] [PubMed] [Google Scholar]
- 70. Yang LE, Sandberg MB, Can AD, Pihakaski‐Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol. 2008;295:F1003–F1016. doi: 10.1152/ajprenal.90235.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang LE, Zhong H, Leong PK, Perianayagam A, Campese VM, McDonough AA. Chronic renal injury‐induced hypertension alters renal NHE3 distribution and abundance. Am J Physiol Renal Physiol. 2003;284:F1056–F1065. doi: 10.1152/ajprenal.00317.2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S3
References 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71


