Abstract
Background
Preeclampsia is pregnancy specific, involving significant maternal endothelial dysfunction. Predictive biomarkers are lacking. We evaluated the biomarker potential, expression, and function of PSG7 (pregnancy‐specific β‐1 glycoprotein 7) and PSG9 (pregnancy‐specific β‐1 glycoprotein 9) in preeclampsia.
Methods and Results
At 36 weeks gestation preceding term preeclampsia diagnosis, PSG7 and PSG9 (in Australian cohorts of n=918 and n=979, respectively) were significantly increased before the onset of term preeclampsia (PSG7, P=0.013; PSG9, P=0.0011). In samples collected at 28 to 32 weeks from those with preexisting cardiovascular disease and at high risk of preeclampsia (Manchester Antenatal Vascular Service, UK cohort, n=235), both PSG7 and PSG9 were also significantly increased preceding preeclampsia onset (PSG7, P<0.0001; PSG9, P=0.0003) relative to controls. These changes were validated in the plasma and placentas of patients with established preeclampsia who delivered at <34 weeks gestation (PSG7, P=0.0008; PSG9, P<0.0001). To examine whether PSG7 and PSG9 are associated with increasing disease severity, we measured them in a cohort from South Africa stratified for this outcome, the PROVE (Preeclampsia Obstetric Adverse Events) cohort (n=72). PSG7 (P=0.0027) and PSG9 (P=0.0028) were elevated among patients who were preeclamptic with severe features (PROVE cohort), but not significantly changed in those without severe features or with eclampsia. In syncytialized first trimester cytotrophoblast stem cells, exposure to TNFα (tumor necrosis factor α) or IL‐6 (interleukin 6) significantly increased the expression and secretion of PSG7 and PSG9. In contrast, when we treated primary endothelial cells with recombinant PSG7 and PSG9, we only observed modest changes in Flt‐1 (FMS‐like tyrosine kinase‐1) expression and Plgf (placental growth factor) expression, and no other effects on proangiogenic/antiangiogenic or endothelial dysfunction markers were observed.
Conclusions
Circulating PSG7 and PSG9 are increased before preeclampsia onset and among those with established disease with their production and release potentially driven by placental inflammation.
Keywords: biomarkers, placenta, preeclampsia, pregnancy, pregnancy specific beta‐1 glycoproteins
Subject Categories: Biomarkers, Pathophysiology
Nonstandard Abbreviations and Acronyms
- Et‐1
endothelin‐1
- flt‐1
fms‐like tyrosine kinase‐1
- hTSC
human trophoblast stem cell
- HUVEC
human umbilical vein endothelial cell
- Icam‐1
intracellular cell adhesion molecule‐1
- MAViS
Manchester Antenatal Vascular Service
- Plgf
placental growth factor
- PROVE
Preeclampsia Obstetric Adverse Events
- PSG7
pregnancy‐specific β‐1 glycoprotein 7
- PSG9
pregnancy‐specific β‐1 glycoprotein 9
- sFlt‐1
soluble fms‐like tyrosine kinase‐1
- Vcam‐1
vascular cell adhesion molecule‐1
- Vegfa
vascular endothelial growth factor A
Clinical Perspective
What Is New?
In numerous large clinical cohorts, plasma PSG7 (pregnancy‐specific β‐1 glycoprotein 7) and PSG9 (pregnancy‐specific β‐1 glycoprotein 9) are consistently increased preceding a diagnosis of preeclampsia and among those with established disease.
PSG7 and PSG9 production and release are potentially driven by placental inflammation.
What Are the Clinical Implications?
Our study suggests that PSG7 and PSG9 have biomarker potential and may have utility if combined with other biomarkers to enhance the early prediction of this serious disease.
Preeclampsia is a serious complication of pregnancy affecting 2% to 8% of all pregnancies and is a leading cause of maternal and neonatal mortality and morbidity worldwide. 1 It originates from poor placentation and is characterized by placental hypoxia, local and systemic inflammation, and widespread maternal endothelial dysfunction. 2 When it occurs, there is excessive release of antiangiogenic and proinflammatory factors from the hypoxic placenta, which cause widespread systemic maternal vascular dysfunction. This then leads to multiorgan injury. To date, the only cure for preeclampsia is delivery of the placenta. 3 , 4
Currently, there are no biomarkers that perform accurately in identifying which pregnancies will develop preeclampsia. Identifying biomarkers that are deranged preceding the clinical diagnosis of preeclampsia has the potential to improve clinical care. It would alert clinicians to patients who are at high risk of developing the condition, allowing for closer clinical observation or even preventive therapies to be implemented.
The PSG (pregnancy‐specific β‐1 glycoprotein) family is a subgroup of the carcinoembryonic antigen family, previously known as Schwangerschafts protein 1. 5 These proteins are encoded by 10 highly conserved PSG genes, Psg1 to Psg9 and Psg11 (Psg10 is a pseudogene), which are clustered in chromosome 19q13.1–13.3. 6 PSGs are the most abundant fetal proteins found in the maternal circulation in late human pregnancy. They are expressed in human embryos as early as the 4‐cell stage and are detectable in the maternal circulation from day 7 after conception. 7 Despite gene cloning and characterization of PSGs, very little is known of their biological functions.
The expression of specific PSG proteins and their role in preeclampsia is poorly explored, with most research investigating PSG1. 8 Abnormal levels of PSG1 have been reported in complications of pregnancy such as preeclampsia, fetal growth restriction, and spontaneous miscarriage. 9 , 10 There have been limited studies of PSG7 and PSG9 proteins in pregnancy. A study from the SCOPE (Screening for Pregnancy Endpoints) consortium using a novel label‐free SRM (Selection Reaction Monitoring) approach identified PSG9 as consistently increased in early‐onset preeclampsia at 15 weeks as well as identifying significant changes in PSG2 and PSG5 with preeclampsia. 1 , 2 , 8 In addition, a 2016 study demonstrated that PSG9, via activation of TGFβ‐1 (transforming growth factor β‐1), may be a potent inducer of immune tolerance, important for early pregnancy maintenance. 11 Interestingly, within the field of oncology, PSG9 has been suggested to be a potential biomarker for colorectal carcinogenesis and hepatocellular cancer. 12 , 13
The purpose of this study was to assess circulating and placental PSG7 and PSG9 levels in several large prospective cohorts as novel biomarkers of established and/or impending preeclampsia. The reason we chose to focus on these 2 proteins is that we identified them in early screening studies as differentially regulated in patients destined to develop preeclampsia. In addition, we aimed to undertake functional studies in vitro to better understand the potential role of these molecules in preeclampsia pathogenesis.
Methods
Data that support the findings of this article are available from the corresponding author upon reasonable request.
Fetal Longitudinal Assessment of Growth Study
The FLAG (Fetal Longitudinal Assessment of Growth) study was undertaken at the Mercy Hospital for Women in Melbourne, Australia, and involved the prospective recruitment of pregnant participants’ blood samples at 28 (27+0 – 29+0 days) and 36 (35+0 – 37+0) weeks gestation as previously described. 14 Whole blood was collected in 9‐mL EDTA tubes. Plasma was stored at −80 °C until the time of sample analysis. The FLAG study was approved by the Mercy Health Research Ethics Committee (Ethics Approval Number R14/12), and written informed consent was obtained from all participants. Preeclampsia was defined using the American College of Obstetricians and Gynecologists (ACOG) guidelines. 15 For this study, PSG7 and PSG9 were measured in plasma samples collected around 36 weeks gestation. See Tables S1 and S2 for patient characteristics. For PSG7, we measured levels in 882 controls and 36 patients who later developed preeclampsia, and for PSG9 we measured levels in 938 controls and 41 patients who later developed preeclampsia.
Manchester Antenatal Vascular Service Cohort
Circulating PSG7 and PSG9 were also measured in plasma samples obtained from the UK Manchester Antenatal Vascular Service (MAViS) clinic as previously described. 14 This study looked at a case cohort of 235 participants whose plasma samples were obtained between 24 and 34 weeks gestation and who were recruited between October 2011 and December 2016. These 235 participants were selected from a biobank of 518 participants. The clinical characteristics have been previously reported. 14 Participants gave written informed consent to donate samples for future research studies. The study was approved by the NRES (National Research Ethics Service) Committee North West 11/NW/0426.
PROVE Cohort
The PROVE (Preeclampsia Obstetric Adverse Events) cohort is a biobank that includes women with preeclampsia and normotensive controls with a special focus on preeclampsia with end‐organ complications such as cerebral oedema; pulmonary oedema; hemolysis, elevated liver enzymes, and low platelet count syndrome; and renal failure as previously described. 14 , 15 This cohort included 72 participants (13 with preeclampsia without severe features, 14 with preeclampsia with severe features, 31 with eclampsia, and 14 normotensive controls) who were recruited from April 2018 to March 2020. The clinical characteristics have been previously reported. 14 Ethical approval was obtained from Stellenbosch University (PROVE; N17/05/048), and all participants gave written informed consent.
Early‐Onset Preeclampsia Plasma Collection
Whole blood was also collected from participants delivering at <34 weeks gestation for preeclampsia (n=46) or from gestation‐matched participants (n=28) who went on to deliver at term without preeclampsia (normotensive). Blood was collected in a 9‐mL EDTA tube. Plasma was stored at −80 °C until analysis. Early‐onset preeclampsia was defined using the ACOG guidelines. 15 Refer to Table S3 for patient characteristics.
Early‐Onset Preeclampsia Placenta Collection
Placentas were obtained from participants who delivered with early‐onset preeclampsia (<34 weeks gestation; n=82) and gestation‐matched normotensive controls (n=20). Control placentas (<34 weeks gestation) were collected from normotensive participants without any evidence of hypertensive disease or fetal growth restriction who were delivered preterm for reasons such as placenta previa or rupture of membranes. Ethics approval was obtained from Mercy Health Human Research Ethics Committee (R11/34). Patients presenting to Mercy Hospital for Women gave written informed consent for the collection of their blood, placentas, and umbilical cords following cesarean delivery.
Placental samples were collected and processed within 30 minutes of delivery. To ensure samples were representative of the entire placenta, samples were taken from each quadrant. Placental pieces were washed immediately in ice‐cold PBS to remove excess blood before being processed to RNAlater stabilization solution and stored at −80 °C for isolation of protein or RNA. Refer to Tables S4 (protein) and Tables S5 (mRNA) for patient characteristics.
Culture and Differentiation of Human Trophoblast Stem Cells
Cytotrophoblast stem cell lines (human trophoblast stem cells [hTSCs]) were imported from the RIKEN BRC through the National BioResource Project of the MEXT/AMED, Japan, and cultured and differentiated into syncytiotrophoblast according to the publication by Okae and colleagues. 17
Treatment of Syncytialized hTSCs With Interleukin 6 and Tumor Necrosis Factor α
Cells were plated at 60 000 cells/well in a 24‐well cell culture plate or 15 000/well in a 96‐well tissue culture plate (CellTiter 96 Aqueous Non‐Radioactive Cell Proliferation Assay [MTS] assay) in syncytial (ST[2D]) medium and incubated at 37 °C, 8% O2, and 5% CO2 for 72 hours to allow syncytialization. Cells were then treated with increasing doses of TNFα (tumor necrosis factor α) 18 or IL‐6 (interleukin 6) 19 at 0, 0.1, and 1 ng/mL for 24 hours. Cells were treated in triplicate and repeated 5 separate times. Conditioned media and cell lysates were collected for subsequent analysis using ELISA and RNA extraction/quantitative real‐time polymerase chain reaction (qRT‐PCR), respectively.
Hypoxic Stimulation of Syncytialized hTSCs
Following seeding, cells were maintained in a humidified incubator at 37 °C, 8% O2, and 5% CO2 for 72 hours to allow complete syncytialization. Cells undergoing hypoxic exposure were then transferred to 1% O2, whereas cells undergoing normoxic exposure remained at 8% O2 for an additional 48 hours. Conditioned media and cell lysates were collected for analysis using ELISA and RNA extraction/qRT‐PCR, respectively.
Isolation and Treatment of Primary Human Umbilical Vein Endothelial Cells
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described. 20 Cells were cultured in M199 media (Life Technologies) containing 100 µg/mL heparin, 1% endothelial cell growth factor (Sigma‐Aldrich), 1% antibiotic‐antimycotic (Life Technologies), and 20% fetal bovine serum at 37 °C, 20% O2, and 5% CO2, and HUVECs were used between the first 1 to 4 passages.
Treatment of Primary HUVECs With Recombinant Human PSG7 and PSG9
Cells were seeded at 20 000/well in a 48‐well cell culture plate or 10 000/well in a 96‐well tissue culture plate (for MTS assay) and incubated overnight at 37 °C, 20% O2, and 5% CO2. Cells were then treated with increasing doses of recombinant PSG7 (Cusabio) or recombinant PSG9 (ProSci Inc) at 0, 0.5, 1, and 2 µg/mL for 24 hours. Cells were treated in triplicate, and the experiment was repeated 5 times. Conditioned media and cell lysates were collected for analysis (ELISA and RNA extraction/qRT‐PCR, respectively).
RNA Extraction
RNA was extracted from placental samples, cytotrophoblast/syncytiotrophoblast, and HUVECs using the GenElute Mammalian Total RNA Miniprep Kit (Sigma‐Aldrich) as per the manufacturer’s instructions. It was quantified using a Nanodrop ND 1000 spectrophotometer (NanoDrop Technologies Inc).
Quantitative Reverse Transcriptase Polymerase Chain Reaction
A total of 1 µg of total placental RNA or 100 ng of total cellular RNA were reverse transcribed to cDNA using the Applied Biosystems High‐Capacity cDNA Reverse Transcription Kit, as per the manufacturer’s guidelines. Gene expressions of Psg7, Psg9, Vcam‐1 (vascular cell adhesion molecule‐1), Icam‐1 (intracellular cell adhesion molecule‐1), Et‐1 (endothelin‐1), Flt‐1 (fms‐like tyrosine kinase‐1), Eng (endoglin), Plgf (placental growth factor), Vegfa (vascular endothelial growth factor A), and Ywhaz (Tyrosine 3‐Monooxygenase/Tryptophan 5‐Monooxygenase Activation Protein Zeta)were quantified by qRT‐PCR on the CFX384 (Bio‐Rad) using Fluorescein amidite (FAM)‐labeled Taqman fast advanced Master Mix (Applied Biosystems) and their specific Taqman Gene expression Assays (Life Technologies). The run conditions were as follows: 95 °C for 20 seconds followed by 40 cycles of 95 °C for 3 seconds and 60 °C for 30 seconds. SYBR qRT‐PCR was carried out to assess the gene expression of sFlt‐1 (soluble fms‐like tyrosine kinase‐1) e15a, sFlt‐1 i13, 21 and Ywhaz on the CFX384 (Bio‐Rad) using Fast SYBR Green Master Mix (Applied Biosystems) and their specific forward and reverse primers. The run conditions were as follows: 95 °C for 20 seconds; 95 °C for 1 second, 60 °C for 20 seconds (40 cycles), and melt curve 65 °C to 95 °C at 0.5 °C increments at 0·05 seconds. All data were normalized to the housekeeping gene (Ywhaz) for in vitro experiments and the geometric mean of topoisomerase‐1 or cyclin‐1 for placental tissues. Samples were run in duplicate and mean Ct (cycle theshold) was used. Results were calibrated against the average Ct of controls and expressed as fold change relative to controls.
ELISA for Measurements of PSG7 and PSG9
Concentrations of PSG7 and PSG9 were measured in plasma, conditioned cell culture media, or cell/tissue lysates using the human PSG7 (MyBioSource) and PSG9 (Aviva Systems Biology) ELISA kits according to the manufacturers’ instructions. Both kits had interassay and intra‐assay precisions of <15%. For the FLAG cohort, some samples fell below the level of detection for the assay, and thus data were included for these samples based on the dilution factor (300× for PSG7 and 500× for PSG9) multiplied by the lowest standard curve value. There were for 16 samples for PSG7 and 36 samples for PSG9.
Statistical Analysis
All in vitro experiments were performed in technical triplicate and repeated at least 3 times. Data obtained were tested for normality using the Anderson–Darling test, D’Agostino and Pearson test, Shapiro–Wilk test, and Kolmogorov–Smirnov test. Statistically appropriate tests were then selected for use based on the data distribution. When 2 groups were analyzed, for unpaired data, either an unpaired t test (parametric) or Mann–Whitney test (nonparametric) was used. For paired data, either a paired t test (parametric) or a Wilcoxon ranked test was used. For ≥3 groups, either 1‐way ANOVA (parametric) or a Kruskal Wallis test was used. To investigate the influence of confounders, regression coefficients were compared in the FLAG cohort using unadjusted and adjusted linear regression models fitted using the natural logarithm of PSG7 or PSG9 levels as the dependent variable and preeclampsia status and baseline maternal characteristics (age, booking body mass index, parity, and smoking status) as the independent variables. A 10% change‐in‐estimate criterion was used to determine whether to include confounders in subsequent analyses. 22 As participants in the MAViS cohort have an underlying vascular disease and both the MAViS and PROVE cohorts were sampled across a wider range of gestations, a series of linear regression models were fitted to determine the change in PSG7 and PSG9 levels with respect to disease status. For each regression model, the natural logarithm of either PSG7 or PSG9 was used as the dependent variable, with disease status, chronic hypertension (MAViS only), renal hypertension (MAViS only), and gestational age at sampling in days as the independent variables. The fitted regression coefficients were then transformed to represent fold change in mean PSG7 and PSG9 levels with respect to controls. All data are expressed as either mean±SEM, median (interquartile range [IQR]), or fold change (95% CI). P<0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 8.4.3 (GraphPad Software, LLC) or RStudio 4.1.0. 23
Results
Circulating PSG7 and PSG9 Are Elevated at 36 Weeks Before the Onset of Term Preeclampsia
We initially measured circulating PSG7 and PSG9 in the plasma of participants at 36 weeks gestation. PSG7 (P=0.013) and PSG9 (P=0.0011) were both significantly elevated in patients before the onset of preeclampsia (n=36 and n=41, respectively) relative to those who did not develop disease (n=882 and n=938, respectively; Figure 1A and 1B). For PSG7, the median concentration was 7.9×105 pg/mL (IQR, 5.2×105–1.7×106 pg/mL) in participants who were destined to develop preeclampsia compared with a median concentration of 6.3×105 pg/mL (IQR, 3.0×105–1.1×106 pg/mL) in those who did not develop disease. For PSG9, the median concentration was 2.1×107 pg/mL (IQR, 1.3×107–3.4×107 pg/mL) in the cohort who later developed preeclampsia compared with a median concentration of 1.4×107 pg/mL (IQR, 7.6×106–2.3×107 pg/mL) in those who did not develop disease. As this cohort represents an unbiased population sample, analysis of the discriminatory power of each biomarker was investigated using the area under the receiver operating characteristic curve demonstrating modest performance for both PSG7 (area under the curve = 0.62; Figure 1C) and PSG9 (area under the curve = 0.65; Figure 1D). To demonstrate that differences in PSG7 and PSG9 levels between preeclampsia cases and controls were not attributed to confounding factors, we fitted unadjusted and adjusted linear regression models, correcting for baseline maternal characteristics (Table S6). This analysis demonstrated that the change‐in‐estimate for the effect of preeclampsia on PSG7 and PSG9 levels between the unadjusted and adjusted models was ≈8.3% for PSG7 and 4.7% for PSG9, meeting the <10% change‐in‐estimate criterion for proceeding with unadjusted analyses.
Figure 1. Circulating PSG7 and PSG9 are increased before diagnosis of preeclampsia.
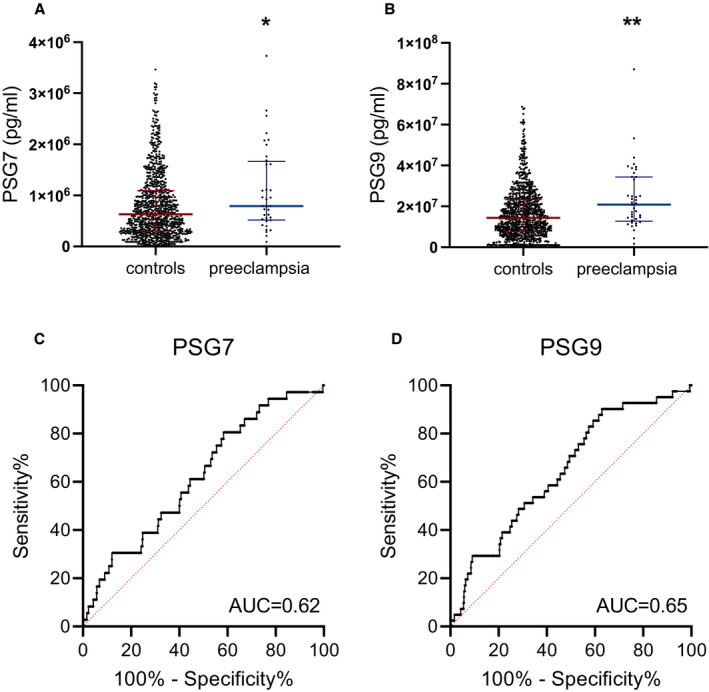
Circulating PSG7 and PSG9 were measured in a large prospective study, the FLAG (Fetal Longitudinal Assessment of Growth) study. These proteins were measured in the plasma of patients preceding their diagnosis with preeclampsia at 36 weeks gestation relative to controls. PSG7 and PSG9 were found to be significantly elevated in the plasma of patients who developed preeclampsia after blood sampling (A and B) relative to controls; individual symbols represent individual patients. The discriminatory power of each biomarker is shown as a receiver operating characteristic curve with the AUC annotated (C and D). Data are expressed as median (interquartile range), and the significance levels determined using a Mann–Whitney U test were *P<0.05 and **P<0.01. AUC indicates area under the curve; PSG7, pregnancy‐specific β‐1 glycoprotein 7; and PSG9, pregnancy‐specific β‐1 glycoprotein 9.
We next sought to validate changes in circulating PSG7 and PSG9 in a high‐risk cohort with samples collected between 24 and 34 weeks preceding clinical diagnosis in Manchester, UK (MAViS cohort). This cohort consisted of patients with preexisting vascular complications, including chronic hypertension and hypertension associated with underlying renal disease. 14 Among women who later developed preeclampsia (n=57), there was a 1.88‐fold change in mean PSG7 levels (95% CI, 1.39–2.55; P<0.0001; Table 1) and a 1.71‐fold change in PSG9 levels (95% CI, 1.28–2.27; P=0.0003; Table 1) relative to 178 patients who did not develop preeclampsia or deliver an infant who was small for gestational age.
Table 1.
Fold Change Expressed as Mean PSG7 and PSG9 Levels With Respect to Controls in the Manchester Antenatal Vascular Service Cohort
| PSG7 | PSG9 | |||||
|---|---|---|---|---|---|---|
| Group | Crude (95% CI) | Adjusted (95% CI) | P value* | Crude (95% CI) | Adjusted (95% CI) | P value* |
| Control, n=57 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||
| Preeclampsia, n=178 | 1.69 (1.25–2.27) | 1.88 (1.39–2.55) | <0.0001 | 1.52 (1.15–2.02) | 1.71 (1.28–2.27) | 0.0003 |
Adjusted analyses were corrected for gestational age at sampling and hypertensive status (renal hypertension or chronic hypertension). PSG7 indicates pregnancy‐specific β‐1 glycoprotein 7; and PSG9, pregnancy‐specific β‐1 glycoprotein 9.
P values for adjusted analyses.
Circulating and Placental PSG7 and PSG9 Are Increased in Women Diagnosed With Preeclampsia
Having demonstrated circulating PSG7 and PSG9 are consistently elevated in patients who later developed preeclampsia, we next sought to assess circulating levels and placental expression in those with established early‐onset disease.
Circulating PSG7 and PSG9 were examined in plasma samples obtained from participants with early‐onset preeclampsia in Melbourne, Australia (<34 weeks gestation). Samples from patients who were preeclamptic (n=46) were compared with samples from patients who were normotensive, matched for gestation at sampling and delivered healthy infants at term (n=28). Circulating PSG7 and PSG9 levels were significantly increased in the plasma from patients with early‐onset preeclampsia relative to gestation‐matched controls (Figure 2A [P=0.0008] and Figure 2B [P<0.0001], respectively). The median for PSG7 was 1.2×106 pg/mL (IQR, 5.2×105–2.3×106 pg/mL) and PSG9 was 2.2×1010 pg/mL (IQR, 1.3×1010–3.7×1010 pg/mL) compared with respective controls (5.1×105 pg/mL [IQR, 1.2×105–9.8×105 pg/mL] for PSG7 and 9.0×109 pg/mL [IQR, 4.4×109–2.0×1010 pg/mL] for PSG9).
Figure 2. Circulating and placental PSG7 and PSG9 are increased in patients diagnosed with preeclampsia.
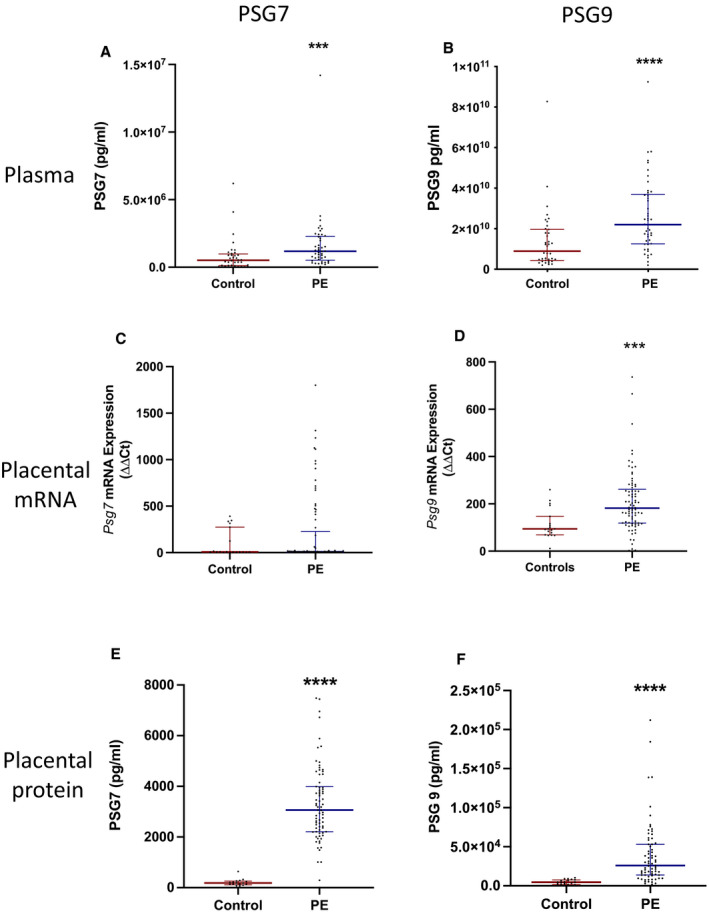
PSG7 and PSG9 proteins were measured in patients with preeclampsia who delivered at <34 weeks gestation. Plasma (A) PSG7 (n=28 control, n=46 preeclampsia) and (B) PSG9 (n=28 control, n=46 preeclampsia) protein concentrations were significantly increased in plasma from women with early‐onset preeclampsia relative to gestation‐matched controls. In placental samples, (C) Psg7 mRNA expression (n=19 control, n=81 preeclampsia) was not significantly different between preeclampsia and control groups, whereas (D) Psg9 mRNA expression (n=19 control, n=81 preeclampsia) was significantly increased in the preeclamptic cohort. Both (E) PSG7 (n=20 control, n=82 preeclampsia) and (F) PSG9 (n=20 control, n=82 preeclampsia) protein concentrations were significantly increased in preeclamptic placental lysates relative to controls. Placental mRNA expression for both PSG7 and PSG9 were normalized to the geometric mean of the reference housekeeping genes, topoisomerase‐1 and cyclin‐1. Individual symbols represent individual patients. Data are expressed as median (interquartile range), and the significance levels determined using a Mann–Whitney U test were ***P<0.001 and ****P<0.0001. PE indicates preeclampsia; PSG7, pregnancy‐specific β‐1 glycoprotein 7; PSG9, pregnancy‐specific β‐1 glycoprotein 9; and ΔΔCT, delta delta CT analysis method.
The mRNA expressions of Psg7 and Psg9 were assessed in 81 early‐onset preeclamptic placentas and 19 gestation‐matched control placentas. There was no significant difference in Psg7 mRNA expression levels between the preeclamptic and control groups (Figure 2C). In contrast, Psg9 mRNA expression was significantly higher in placentas from women with preeclampsia compared with controls (Figure 2D; P=0.0002).
Next, PSG7 and PSG9 proteins were measured in placental lysates obtained from 82 women with early‐onset preeclampsia and 20 controls where the median gestation at sampling was matched to the preeclamptic cohort. Both PSG7 and PSG9 placental protein concentrations were significantly increased in preeclamptic placental lysates relative to control lysates (Figure 2E and 2F; P<0.0001 for both).
Finally, circulating PSG7 and PSG9 were examined in the PROVE cohort to assess their association with different severities of disease, as defined by the ACOG criteria. 15 Samples were collected from participants at gestations ranging from 21 to 41 weeks who had either (1) preeclampsia without severe features, (2) preeclampsia with severe features, or (3) eclampsia or from normotensive controls. In the PROVE cohort, no significant change was observed in patients with preeclampsia without severe features (for PSG7, mean fold change=1.00 [95% CI, 0.45–2.25], P=1.00; for PSG9, mean fold change=1.04 [95% CI, 0.48–2.25], P=0.92; Table 2) relative to controls. However, in patients with preeclampsia with severe features, PSG7 and PSG9 were significantly elevated with a 3.72‐fold change in mean PSG7 levels (95% CI, 1.60–8.65; P=0.0027; Table 2) and a 3.50‐fold change in PSG9 levels relative to controls (95% CI, 1.56–7.85; P=0.0028; Table 2). In patients who were eclamptic, no significant change in PSG7 or PSG9 was demonstrated relative to controls: mean fold changes of 1.66 (95% CI, 0.83–3.32; P=0.15) and 1.65 (95% CI, 0.85–3.19; P=0.14; Table 2) for PSG7 and PSG9 levels, respectively.
Table 2.
Fold Change Expressed as Mean PSG7 and PSG9 Levels With Respect to Controls in the Preeclampsia Obstetric Adverse Events Cohort Grouped by Disease Severity
| PSG7 | PSG9 | |||||
|---|---|---|---|---|---|---|
| Group | Crude (95% CI) | Adjusted (95% CI) | P value* | Crude (95% CI) | Adjusted (95% CI) | P value* |
| Control, n=14 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||
| Preeclampsia without severe features, n=13 | 0.93 (0.40–2.15) | 1.00 (0.45–2.25) | 1.00 | 0.97 (0.44–2.16) | 1.04 (0.48–2.25) | 0.92 |
| Preeclampsia with severe features, n=14 | 2.57 (1.12–5.86) | 3.72 (1.60–8.65) | 0.0027 | 2.49 (1.13–5.47) | 3.50 (1.56–7.85) | 0.0028 |
| Eclampsia, n=31 | 1.37 (0.68–2.77) | 1.66 (0.83–3.32) | 0.15 | 1.38 (0.71–2.70) | 1.65 (0.85–3.19) | 0.14 |
Adjusted analyses were corrected for gestational age at sampling. PSG7 indicates pregnancy‐specific β‐1 glycoprotein 7; and PSG9, pregnancy‐specific β‐1 glycoprotein 9.
P values for adjusted analyses.
Effect of Hypoxia and Inflammatory Insult on PSG7 and PSG9 Expression and Secretion in Syncytialized Cytotrophoblast Stem Cells
Preeclampsia is associated with placental hypoxia 3 ; we thus set out to determine whether placental hypoxia would alter PSG7 and PSG9 expression and secretion. Because PSGs are known to be synthesized within syncytiotrophoblast, for this experiment syncytialized hTSCs were used. When syncytialized hTSCs were exposed to hypoxia (1% O2), both PSG7 and PSG9 secretion were significantly reduced (Figure 3A [P=0.0079] and Figure 3C [P=0.0079]) compared with cells cultured under normoxic (8% O2) conditions. In contrast, relative mRNA expression of Psg7 was significantly increased under hypoxic conditions (Figure 3B [P=0.0079]), whereas no significant change in Psg9 mRNA expression was observed (Figure 3D). These data demonstrate that hypoxic conditions likely downregulate PSG7 and PSG9 secretion in first‐trimester syncytiotrophoblast, suggesting that the elevated PSG7 and PSG9 observed in preeclamptic placentas (and possibly plasma) is unlikely to be induced by placental hypoxia.
Figure 3. The effect of hypoxia and inflammation on PSG7 and PSG9 mRNA expression and secretion in syncytialized cytotrophoblast stem cells.
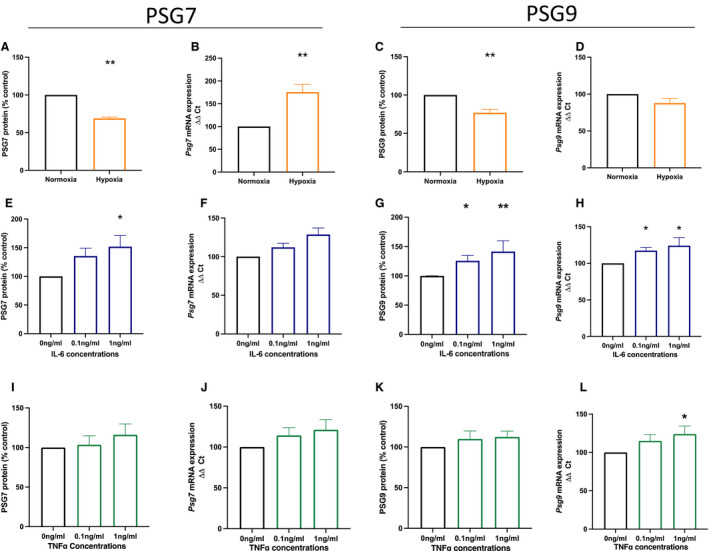
Syncytiotrophoblast cells were exposed to either (A through D) hypoxic conditions or (E through L) inflammatory stimuli. When cells were exposed to hypoxia (1% oxygen; orange), both (A) PSG7 and (C) PSG9 protein secretion were significantly reduced compared with the normoxic (8% oxygen; black) group. Psg7 mRNA expression was significantly increased in syncytiotrophoblast cells cultured under hypoxic conditions (B); however, there was no significant difference in Psg9 mRNA expression (D). Next, syncytiotrophoblast cells were treated with increasing doses of either IL‐6 (E through H; blue) or TNFα (I through L; green; 0 ng/mL, 0.1 ng/mL, 1 ng/mL). Treatment with IL‐6 significantly increased PSG7 secretion from syncytiotrophoblast cells with the 1 ng/mL dose (E), whereas there was no significant change in Psg7 mRNA expression (F). Treatment with 0.1 ng/mL and 1 ng/mL IL‐6 significantly increased both PSG9 secretion (G) and mRNA expression (H) in syncytiotrophoblast cells. In TNFα‐treated syncytiotrophoblast cells, there was no significant change in PSG7 (I) and PSG9 (K) protein secretion and Psg7 mRNA expression (J), whereas treatment with 1 ng/mL TNFα significantly increased Psg9 mRNA expression (L). Protein levels were normalized as percentage of controls within the experiment, and mRNA expression was normalized to the geometric mean of housekeeping genes. All experiments were repeated 5 times with triplicate repeats. Data are expressed as mean±SEM, and the significance levels determined using a Mann–Whitney U test for 2 groups or a Kruskal–Wallis test with multiple comparisons for ≥3 groups were *P<0.05 and **P<0.01. IL‐6 indicates interleukin‐6; PSG7, pregnancy‐specific β‐1 glycoprotein 7; PSG9, pregnancy‐specific β‐1 glycoprotein 9; TNFα, tumor necrosis factor α; and ΔΔCT, delta delta CT analysis method.
Because preeclampsia is associated with systemic and placental inflammation, syncytialized cytotrophoblast stem cells were treated with increasing doses of proinflammatory cytokines (IL‐6 and TNFα) to assess whether these cytokines regulate placental PSG7 and PSG9 secretion or expression. Importantly, both IL‐6 and TNFα have been identified as significantly increased in the circulation of patients with preeclampsia. 24 IL‐6 treatment resulted in significantly increased PSG7 secretion at a dose of 1 ng/mL (Figure 3E [P=0.021 for trend and P=0.018 for 1 ng/mL dose]), whereas no significant changes in Psg7 mRNA expression were observed (Figure 3F [P=0.10 for trend]). Interestingly, IL‐6 treatment at both 0.1 ng/mL and 1 ng/mL concentrations significantly increased both PSG9 secretion (Figure 3G [P=0.0021 for trend, P=0.027 for 0.1 ng/mL, and P=0.0094 for 1 ng/mL]) and mRNA expression (Figure 3H [P=0.0024 for trend, P=0.020 for 0.1 ng/mL, and P=0.011 for 1 ng/mL]).
Next, syncytiotrophoblast cells were treated with increasing doses of TNFα. Data shown in Figure 3I and 3J show no significant effects of TNFα on PSG7 secretion (P=0.35 for trend) or mRNA expression (P=0.17 for trend). Similarly, PSG9 secretion was unchanged following TNFα treatment (Figure 3K [P=0.48 for trend]), whereas a modest but significant increase in Psg9 mRNA expression was observed at a concentration of 1 ng/mL TNFα (Figure 3L [P=0.03 for trend, P=0.026 for 1 ng/mL]).
Overall, these data suggest that these 2 proinflammatory cytokines may contribute modestly to elevations in syncytiotrophoblast expression and secretion of PSG7 and PSG9 from the placenta.
Effect of PSG7 and PSG9 on Proangiogenic and Antiangiogenic Markers in Endothelial Cells
Preeclampsia is characterized by widespread maternal endothelial cell dysfunction believed to culminate from increased release of placental factors. 25 , 26 Because PSGs have been shown to stimulate the release of anti‐inflammatory cytokines and proangiogenic factors in cancer, 27 we assessed the effect of recombinant PSG7 and recombinant PSG9 (at levels similar to those found in the maternal circulation in preeclampsia) on proangiogenic and antiangiogenic mRNA expression in primary HUVECs. We measured antiangiogenic molecule Flt‐1, the sFlt‐1 variants i13 and e15a, 28 , 29 and endoglin 26 given the many reports on these molecules being deranged in preeclampsia and a potential source being the endothelium. Similarly, we measured the proangiogenic molecules PlGF and VEGFA because these are both reported as reduced in preeclampsia. 30
PSG7‐treated HUVECs had a modest but significant increase in Flt‐1 (Figure 4A [P=0.0079]) mRNA expression, whereas no significant changes in sFlt‐1 e15a (Figure 4B), sFlt‐1 i13 (Figure 4C), Eng (Figure 4D), Plgf (Figure 4E), or VegfA (Figure 4F) mRNA expression were observed.
Figure 4. The effect of PSG7 and PSG9 on proangiogenic and antiangiogenic factors in primary HUVECs.
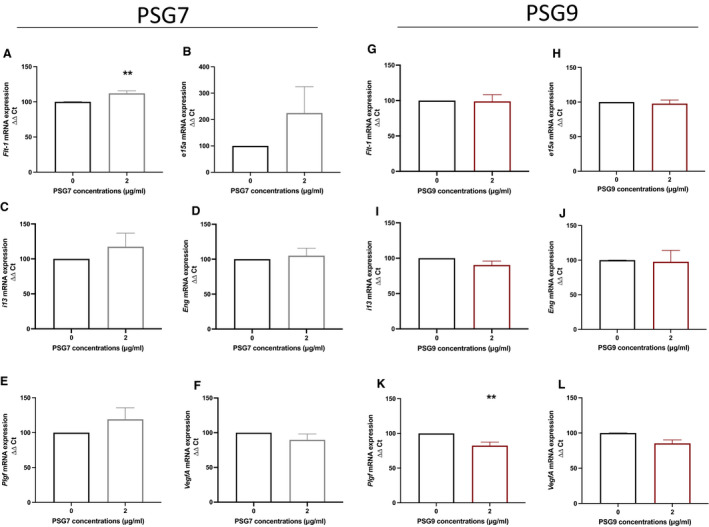
Primary HUVECs were treated with a 2‐µg/mL dose of either recombinant PSG7 (gray) or recombinant PSG9 (maroon), and the effect on Flt‐1, sFlt‐1 e15a, sFlt‐1 i13, Eng, Plgf, and VegfA mRNA expression was assessed. Treatment with PSG7 significantly increased (A) Flt‐1 mRNA expression, whereas PSG7 had no significant effect on (B) sFlt‐1 e15a, (C) sFlt‐1 i13, (D) Eng, (E) Plgf, or (F) VegfA mRNA expression. Furthermore, there was no significant change in (G) Flt‐1, (H) sFlt‐1 e15a, (I) sflt‐1 i13, (J) Eng, or (L) VegfA expression in PSG9‐treated HUVECs. However, (K) Plgf was significantly reduced in PSG9‐treated HUVECs. mRNA expression was normalized to the reference housekeeper Ywhaz. All experiments were repeated 5 times, and each treatment was run in triplicate. Data are expressed as mean±SEM, and the significance level determined using a Mann–Whitney U test was **P<0.01. Eng indicates endoglin; Flt‐1, FMS‐like tyrosine kinase‐1; HUVEC, human umbilical vein endothelial cell; Plgf, placental growth factor; PSG7, pregnancy‐specific β‐1 glycoprotein 7; PSG9, pregnancy‐specific β‐1 glycoprotein 9; sFlt‐1, soluble fms‐like tyrosine kinase‐1; VegfA, vascular endothelial growth factor a; Ywhaz, Tyrosine 3‐Monooxygenase/Tryptophan 5‐Monooxygenase Activation Protein Zeta; and ΔΔCT, delta delta CT analysis method.
Treatment of HUVECs with recombinant PSG9 resulted in a modest reduction in Plgf mRNA expression (Figure 4K [P=0.0079]), whereas no significant changes in Flt‐1 (Figure 4G), sFlt ‐1 e15a (Figure 4H), sFlt‐1 i13 (Figure 4I), Eng (Figure 4J), or VegfA (Figure 4L) mRNA expression were observed.
Recombinant PSG7 and PGS9 Do Not Alter Markers of Endothelial Dysfunction
VCAM‐1, ICAM‐1, and ET‐1 are molecules upregulated in association with the endothelial dysfunction characteristic of preeclampsia. VCAM‐1 and ICAM‐1 are cell adhesion molecules that mediate adhesion of leukocytes, whereas ET‐1 is a potent vasoconstrictor. 31 , 32 Increasing doses of PSG7 had no significant effect on Vcam‐1 (P value for trend=0.24) or Icam‐1 (P value for trend=0.23) mRNA expression in HUVECs (Figure 5A and 5B) compared with vehicle control. However, treatment with a 2‐µg/mL concentration of PSG7 induced a modest but significant reduction in Et‐1 mRNA expression in HUVECs relative to control (Figure 5C [P value for trend=0.028, P=0.015 for 2 µg/mL]).
Figure 5. The effect of PSG7 and PSG9 on markers of endothelial dysfunction in primary HUVECs.
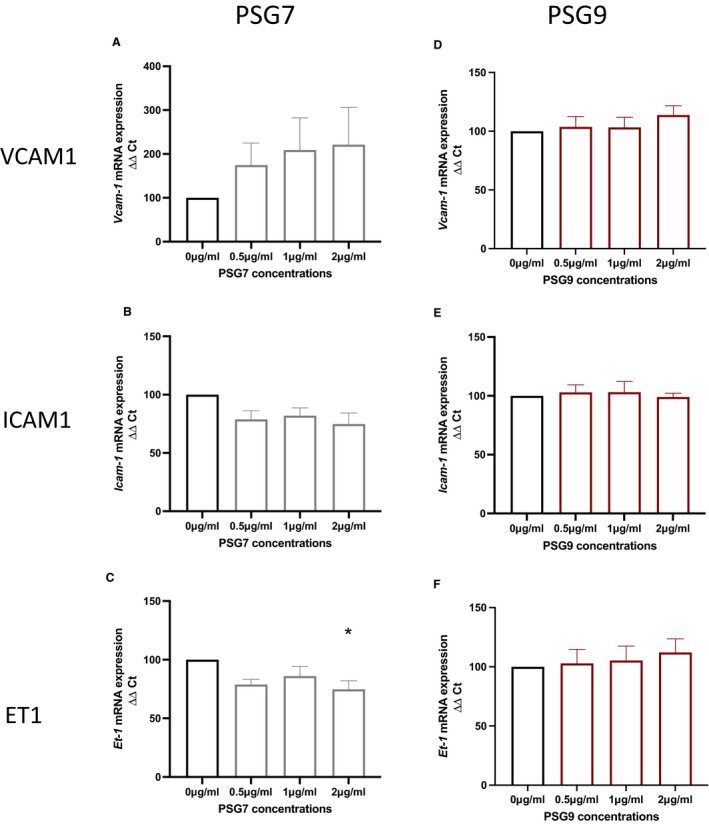
Primary HUVECs were treated with recombinant PSG7 (gray) or recombinant PSG9 (maroon; 0 µg/mL [control], 0.5, 1, or 2 µg/mL) and the effect on endothelial dysfunction markers was assessed. In PSG7‐treated HUVECs, no significant effect on Vcam‐1 and Icam‐1 expression in HUVECS was observed (A and B), whereas PSG7 significantly decreased Et‐1 mRNA expression (C). In PSG9‐treated HUVECs, no significant effects of PSG9 on Vcam‐1, Icam‐1, or Et‐1 mRNA expression was observed (D through F). mRNA expression was normalized to the housekeeper Ywhaz. All experiments were repeated 5 times, and each treatment was run in triplicate. Data are expressed as mean±SEM, and the significance level determined using a Kruskal–Wallis test with multiple comparisons was *P<0.05. Et‐1 indicates endothelin‐1; HUVEC, human umbilical vein endothelial cell; Icam‐1, intracellular cell adhesion molecule‐1; PSG7, pregnancy‐specific β‐1 glycoprotein 7; PSG9, pregnancy‐specific β‐1 glycoprotein 9; Vcam‐1, vascular cell adhesion molecule‐1; Ywhaz, Tyrosine 3‐Monooxygenase/Tryptophan 5‐Monooxygenase Activation Protein Zeta; and ΔΔCT, delta delta CT analysis method.
Increasing doses of PSG9 had no effect on Vcam‐1 (P=0.49), Icam‐1 (P=0.90), or Et‐1 (P=0.75) mRNA expression in HUVECs (Figure 5D through 5F) relative to control.
Discussion
This study identified PSG7 and PSG9 as elevated in plasma both before and after a diagnosis of preeclampsia, with some evidence of greater elevation among those with severe disease. In vitro studies suggested that IL‐6 and TNFα may induce PSG7 and PSG9 expression and transcription in syncytiotrophoblast cells.
PSGs are molecules that are highly expressed in the placenta relative to other tissues. 7 , 33 To date, there has been little study on PSGs and their role in preeclampsia, with most research having focused on PSG1. A major aim of this study was to examine the circulating levels of PSG7 and PSG9 in preeclampsia (both before and after diagnosis). Indeed, both were consistently increased before the onset of preeclampsia and in those with established early‐onset preeclampsia or preeclampsia with severe features/end‐organ damage. Our analyses in the MAViS cohort demonstrated that PSG7 and PSG9 were elevated even in the high‐risk cohort patients with preexisting vascular complications, such as chronic hypertension and hypertension associated with underlying renal disease. Data analyses in the PROVE cohort suggested that circulating PSG7 and PSG9 increased with disease severity and is significantly elevated in patients with preeclampsia with severe features. Interestingly, no significant change in PSG7 and PSG9 levels was observed in women with eclampsia. Eclampsia refers to the occurrence of tonic‐clonic seizures in the presence of preeclampsia, but in the absence of any other reason for the cause of those seizures. 34 Although eclampsia if often defined as a severe complication arising from preeclampsia, it can occur in the absence of hypertension. 35 The reasons why PSG7 and PSG9 were not deranged in eclampsia in the PROVE cohort is not known but highlights the complexity of the disease process. Indeed, it may be that PSG7 and PSG9 contribute to the pathophysiological pathways that lead to other end‐organ complications such as hemolysis, elevated liver enzymes, and low platelet count syndrome, but not to those leading to cerebral complications that result in eclampsia. This needs further investigation so that we can characterize the biomarker profile in those who develop preeclampsia with severe features, relative to eclampsia.
In accord with findings in the plasma, PSG7 and PSG9 were also elevated in the placentas from patients delivering with early‐onset preeclampsia. These findings were suggestive of the placenta being a major source of the elevated circulating levels present preceding diagnosis and in established preeclampsia, supported by our studies showing secretion from placental cells in vitro.
Although the data showed a strong association between PSG7 and PSG9 and preeclampsia, little is known about the regulation of PSGs. Placental hypoxia and inflammation are 2 major characteristics of preeclampsia. 36 , 37 Our new data suggested that placental inflammation may be a contributor to increased placental and secreted levels, whereas hypoxia reduced their expression. This is interesting given our findings that PSG7 and PSG9 were consistently elevated in the plasma of patients with early‐onset preeclampsia and term disease, but not in samples from those with eclampsia. Earlier studies have shown that PSGs play a role in modulating the maternal immune system, demonstrating inhibitory effects on phytohemagglutinin or allogeneically stimulated lymphocytes. 38 , 39 There have also been studies suggesting that they promote early angiogenesis in the developing placenta. 40 , 41 , 42 Work from Shanley et al suggested that both PSG1 and PSG9 regulate platelet–fibrinogen interactions and that PSG9 has antiplatelet activity. 42 This is interesting given our finding of increased PSG7 and PSG9 in patients with preeclampsia with severe features and suggests that high levels of circulating PSG9 might contribute to the maternal syndrome.
Because PSGs are a family of highly conserved proteins, it is intriguing to discover significant changes in PSG7 and PSG9, although in previous studies we have found no change in PSG1 at 36 weeks gestation in those destined to develop term preeclampsia (T.J. Kaitu'u‐Lino et al, unpublished data, 2018). Prior work has also suggested that PSG2 and PSG5 may be deranged as early as 15 weeks gestation in those destined to develop preeclampsia. 8 Although highly conserved with shared biological functions, it appears that some PSG family members might be deranged in preeclampsia, whereas others are not. Thus, further careful studies to understand the regulation of the PSG family members is needed to better understand their role in disease pathogenesis and their potential as disease biomarkers.
Endothelial dysfunction is characterized by increased adhesion molecules and endothelial cell permeability. 43 , 44 PSGs are known to be immunomodulators that secrete anti‐inflammatory cytokines and regulate T cell function. 45 , 46 We hypothesized that PSG7 and PSG9 may increase markers of endothelial dysfunction and might also modulate proangiogenic or antiangiogenic factors as has been observed in colon cancer. 47 In contrast to this hypothesis, direct effects of PSG7 or PSG9 on markers of endothelial dysfunction were not observed. We did, however, observe modest but significant reductions in Plgf mRNA expression in PSG9‐treated HUVECs, whereas PSG7 modestly increased Flt‐1 expression. In future studies, exploration around whether these same alterations in angiogenic and antiangiogenic markers occur in placental cells with high levels of PSG7 or PSG9 would be of great interest to aid in elucidating whether PSG7 and PSG9 are disease drivers or perhaps elevated bystanders. Furthermore, investigations into other aspects of endothelial dysfunction such as effects on the nitric oxide pathway (vasodilation/constriction) and vascular reactivity studies may provide further insight into whether high circulating levels of PSG7 and PSG9 contribute to the pathogenesis of preeclampsia.
A significant strength of this study was our use of 3 independent, well‐characterized international cohorts that enabled us to look at both term and early‐onset disease in high‐risk and unselected populations and in different subtypes of pre/eclampsia. This represents a robust and comprehensive analysis. However, we were limited to using commercially available research grade ELISAs, and thus future development of highly specific, clinical‐grade ELISAs would be needed if these proteins were to be considered for clinical use in combination with other biomarkers.
In conclusion, this study provided consistent data in numerous cohorts indicating that circulating PSG7 and PSG9 are elevated in preeclampsia. However, the pathways regulating elevated PSG7 and PSG9 expression and secretion remain unclear with inflammation as a potential contributor.
Sources of Funding
Funding for this work was provided by the National Health and Medical Research Council (1065854, 1183854, 116071, 2000732), the Norman Beischer Medical Research Foundation, Australian Government Research Training Program Scholarship, and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists Taylor Hammond Scholarship to Dr MacDonald and National Health and Medical Research Council fellowships to Prof Kaitu’u‐Lino (1159261), Prof Hannan (1146128), and Prof Tong (1136418). The funders played no role in study design or analysis.
Disclosures
None.
Supporting information
Tables S1–S6
Acknowledgments
We thank Sally Beard and Natalie Binder for their technical assistance. We thank Valerie Kyritsis, Kirsten Dane, Anna Middleton, Gabrielle Pell, Rachel Murdoch, Genevieve Christophers, Elizabeth Lockie, and Emma McLaughlin for their assistance in recruiting and characterizing participants. We also thank the pathology, health information services, and prenatal clinic staff at the Mercy Hospital for Women for their assistance in conducting this research and the women for agreeing to participate. We thank the staff and patients from Tygerberg Hospital South Africa and the Manchester Antenatal Vascular Service clinic for their contribution and participation in this work. Cytotrophoblast stem cell lines (human trophoblast stem cells) were imported from the RIKEN BRC through the National BioResource Project of the MEXT/AMED, Japan.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024536
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 2. Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33–46. doi: 10.1016/j.semnephrol.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gathiram P, Moodley J. Pre‐eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 2016;27:71. doi: 10.5830/CVJA-2016-009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Redman C, Sargent I. Placental stress and pre‐eclampsia: a revised view. Placenta. 2009;30:38–42. doi: 10.1016/j.placenta.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 5. Brümmendorf T, Rathjen FG. Cell adhesion molecules. 1: immunoglobulin superfamily. Protein Profile. 1994;1:951–1058. [PubMed] [Google Scholar]
- 6. Thompson J, Koumari R, Wagner K, Barnert S, Schleussner C, Schrewe H, Zimmermann W, Müller G, Schempp W, Zaninetta D, et al. The human pregnancy‐specific glycoprotein genes are tightly linked on the long arm of chromosome 19 and are coordinately expressed. Biochem Biophys Res Comm. 1990;167:848–859. doi: 10.1016/0006-291X(90)92103-7 [DOI] [PubMed] [Google Scholar]
- 7. Gordon Y, Jeffrey D, Grudzinskas J, Chard T, Letchworth A. Concentrations of pregnancy‐specific β1‐glycoprotein in maternal blood in normal pregnancy and in intrauterine growth retardation. Lancet. 1977;309:331–333. doi: 10.1016/S0140-6736(77)91135-7 [DOI] [PubMed] [Google Scholar]
- 8. Blankley RT, Fisher C, Westwood M, North R, Baker PN, Walker MJ, Williamson A, Whetton AD, Lin W, McCowan L, et al. A label‐free selected reaction monitoring workflow identifies a subset of pregnancy specific glycoproteins as potential predictive markers of early‐onset pre‐eclampsia. Mol Cell Proteomics. 2013;12:3148–3159. doi: 10.1074/mcp.M112.026872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grudzinskas J, Gordon Y, Menabawey M, Lee J, Wadsworth J, Chard T. Identification of high‐risk pregnancy by the routine measurement of pregnancy‐specific β1‐glycoprotein. Am J Obstet Gynecol. 1983;147:10–12. doi: 10.1016/0002-9378(83)90075-3 [DOI] [PubMed] [Google Scholar]
- 10. Temur M, Serpim G, Tuzluoğlu S, Taşgöz FN, Şahin E, Üstünyurt E. Comparison of serum human pregnancy‐specific beta‐1‐glycoprotein 1 levels in pregnant women with or without preeclampsia. J Obstet Gynaecol. 2019;1–5. doi: 10.1080/01443615.2019.1679734 [DOI] [PubMed] [Google Scholar]
- 11. Jones K, Ballesteros A, Mentink‐Kane M, Warren J, Rattila S, Malech H, Kang E, Dveksler G. PSG9 stimulates increase in FoxP3+ regulatory T‐cells through the TGF‐beta1 pathway. PLoS One. 2016;11:e0158050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salahshor S, Goncalves J, Chetty R, Gallinger S, Woodgett JR. Differential gene expression profile reveals deregulation of pregnancy specific β1 glycoprotein 9 early during colorectal carcinogenesis. BMC Cancer. 2005;5:66. doi: 10.1186/1471-2407-5-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rong W, Yang L, Yin L, Gao Y, Xiao T, Cheng S. PSG9 promotes angiogenesis by stimulating VEGFA production and is associated with poor prognosis in hepatocellular carcinoma. Sci China Life Sci. 2017;60:528–535. doi: 10.1007/s11427-016-0226-7 [DOI] [PubMed] [Google Scholar]
- 14. Cruickshank T, MacDonald TM, Walker SP, Keenan E, Dane K, Middleton A, Kyritsis V, Myers J, Cluver C, Hastie R, et al. Circulating growth differentiation factor 15 is increased preceding preeclampsia diagnosis: implications as a disease biomarker. J Am Heart Assoc. 2021;10:e020302. doi: 10.1161/JAHA.120.020302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Obstetricians and Gynecologists . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 16. Bergman L, Bergman K, Langenegger E, Moodley A, Griffith‐Richards S, Wikström J, Hall D, Joubert L, Herbst P, Schell S, et al. PROVE—Pre‐eclampsia obstetric adverse events: establishment of a biobank and database for pre‐eclampsia. Cells. 2021;10:959. doi: 10.3390/cells10040959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22:50–63. e56. doi: 10.1016/j.stem.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 18. Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor‐alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor‐1 in first‐trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. [DOI] [PubMed] [Google Scholar]
- 19. Meisser A, Cameo P, Islami D, Campana A, Bischof P. Effects of interleukin‐6 (IL‐6) on cytotrophoblastic cells. Mol Hum Reprod. 1999;5:1055–1058. doi: 10.1093/molehr/5.11.1055 [DOI] [PubMed] [Google Scholar]
- 20. Brownfoot F, Hannan N, Onda K, Tong S, Kaitu'u‐Lino T. Soluble endoglin production is upregulated by oxysterols but not quenched by pravastatin in primary placental and endothelial cells. Placenta. 2014;35:724–731. doi: 10.1016/j.placenta.2014.06.374 [DOI] [PubMed] [Google Scholar]
- 21. Palmer KR, Kaitu’u‐Lino TJ, Hastie R, Hannan NJ, Ye L, Binder N, Cannon P, Tuohey L, Johns TG, Shub A, et al. Placental‐specific sFLT‐1 e15a protein is increased in preeclampsia, antagonizes vascular endothelial growth factor signaling, and has antiangiogenic activity. Hypertension. 2015;66:1251–1259. doi: 10.1161/HYPERTENSIONAHA.115.05883 [DOI] [PubMed] [Google Scholar]
- 22. Maldonado G, Greenland S. Simulation study of confounder‐selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813 [DOI] [PubMed] [Google Scholar]
- 23. Team R . Rstudio: integrated development environment for R. RStudio; 2021.
- 24. Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x [DOI] [PubMed] [Google Scholar]
- 25. Maynard SE, Min J‐Y, Merchan J, Lim K‐H, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–658. doi: 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venkatesha S, Toporsian M, Lam C, Hanai J‐I, Mammoto T, Kim YM, Bdolah Y, Lim K‐H, Yuan H‐T, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429 [DOI] [PubMed] [Google Scholar]
- 27. Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23:742–750. doi: 10.1053/plac.2002.0865 [DOI] [PubMed] [Google Scholar]
- 28. Palmer KR, Tong S, Kaitu'u‐Lino TJ. Placental‐specific sFLT‐1: role in pre‐eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis. Mol Hum Reprod. 2017;23:69–78. [DOI] [PubMed] [Google Scholar]
- 29. Levine RJ, Maynard SE, Qian C, Lim K‐H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884 [DOI] [PubMed] [Google Scholar]
- 30. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149 [DOI] [PubMed] [Google Scholar]
- 31. Nishikawa S, Miyamoto A, Yamamoto H, Ohshika H, Kudo R. The relationship between serum nitrate and endothelin‐1 concentrations in preeclampsia. Life Sci. 2000;67:1447–1454. doi: 10.1016/S0024-3205(00)00736-0 [DOI] [PubMed] [Google Scholar]
- 32. Sanchez‐Aranguren LC, Prada CE, Riano‐Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 2014;5:372. doi: 10.3389/fphys.2014.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin TM, Halbert SP, Spellacy WN. Measurement of pregnancy‐associated plasma proteins during human gestation. J Clin Invest. 1974;54:576–582. doi: 10.1172/JCI107794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7 [DOI] [PubMed] [Google Scholar]
- 35. Sibai B, Dekker G, Kupferminc M. Pre‐eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2 [DOI] [PubMed] [Google Scholar]
- 36. Hung T‐H, Charnock‐Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor‐α from human placental tissues induced by hypoxia‐reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164:1049–1061. doi: 10.1016/S0002-9440(10)63192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x [DOI] [PubMed] [Google Scholar]
- 38. Majumdar S, Bapna BC, Mapa MK, Gupta AN, Devi PK, Subrahmanyam D. Pregnancy specific proteins: suppression of in vitro blastogenic response to mitogen by these proteins. Int J Fertil. 1982;27:66–69. [PubMed] [Google Scholar]
- 39. Harris SJ, Anthony FW, Jones DB, Masson GM. Pregnancy‐specific‐beta 1‐glycoprotein: effect on lymphocyte proliferation in vitro. J Reprod Immunol. 1984;6:267–270. [DOI] [PubMed] [Google Scholar]
- 40. Ha CT, Wu JA, Irmak S, Lisboa FA, Dizon AM, Warren JW, Ergun S, Dveksler GS. Human pregnancy specific beta‐1‐glycoprotein 1 (PSG1) has a potential role in placental vascular morphogenesis. Biol Reprod. 2010;83:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lisboa FA, Warren J, Sulkowski G, Aparicio M, David G, Zudaire E, Dveksler GS. Pregnancy‐specific glycoprotein 1 induces endothelial tubulogenesis through interaction with cell surface proteoglycans. J Biol Chem. 2011;286:7577–7586. doi: 10.1074/jbc.M110.161810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shanley DK, Kiely PA, Golla K, Allen S, Martin K, O'Riordan RT, Ball M, Aplin JD, Singer BB, Caplice N, et al. Pregnancy‐specific glycoproteins bind integrin alphaiibbeta3 and inhibit the platelet‐fibrinogen interaction. PLoS One. 2013;8:e57491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–969. doi: 10.1038/ajh.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Austgulen R, Lien E, Vince G, Redman CW. Increased maternal plasma levels of soluble adhesion molecules (ICAM‐1, VCAM‐1, E‐selectin) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;71:53–58. doi: 10.1016/S0301-2115(96)02647-4 [DOI] [PubMed] [Google Scholar]
- 45. Bebo BF Jr, Dveksler GS. Evidence that pregnancy specific glycoproteins regulate T‐cell function and inflammatory autoimmune disease during pregnancy. Curr Drug Targets‐Inflamm Allergy. 2005;4:231–237. [DOI] [PubMed] [Google Scholar]
- 46. Fialova L, Kohoutova B, Peliskova Z, Malbohan I, Mikulikova L. Serum levels of trophoblast‐specific beta‐1‐globulin (SP1) and alpha‐1‐fetoprotein (AFP) in pregnant women with rheumatoid arthritis. Cesk Gynekol. 1991;56:166–170. [PubMed] [Google Scholar]
- 47. Yang L, Hu S, Tan J, Zhang X, Yuan W, Wang Q, Xu L, Liu J, Liu Z, Jia Y, et al. Pregnancy‐specific glycoprotein 9 (PSG9), a driver for colorectal cancer, enhances angiogenesis via activation of SMAD4. Oncotarget. 2016;7:61562. doi: 10.18632/oncotarget.11146 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


