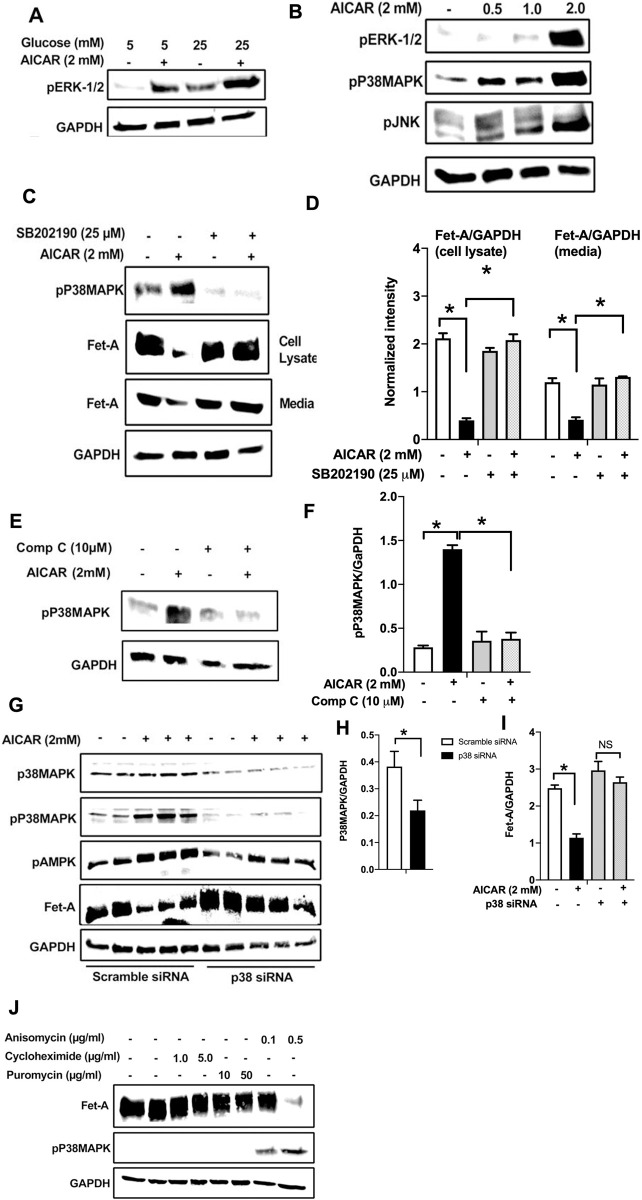
Fig 3. AMPK activation downregulates Fet-A expression through p38 MAPK.
[A] HepG2 cells were incubated with either low- or high-glucose in the absence or presence of AICAR for 12hr and used for Western blot analysis for ERK1/2 phosphorylation expression (n = 3). [B] HepG2 cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for ERK1/2, p38MAPK and JNK phosphorylation (n = 3). [C] Cells were treated with p38 MAPK inhibitor [SB202190, n = 4] before treatment of AICAR for 12 hr. Cell lysate or media were analyzed by Western blotting for indicated proteins and [D] Fet-A levels, as a ratio to GAPDH were determined. [E] Cells were treated with AMPK inhibitor [Comp C, n = 4] before treatment of AICAR for 12hr. Cell lysates were analyzed by Western blotting for phosphorylated p38 MAPK (pP38MAPK) and [F] pP38MAPK levels, as a ratio to GAPDH were determined. [G] Knockdown of p38 MAPK was performed using MAPK14 [p38 MAPK] small interfering RNA [siRNA] in HepG2 cells. Following AICAR treatment for 12 h, cell lysates were analyzed by Western blotting for expression of p38 MAPK, phosphorylated p38 MAPK, Fet-A, and pAMPK. [H] Efficiency of p38MAPK siRNA in HepG2 cells were determined by immunoblotting transfected cells for p38MAPK and levels were expressed as a ratio to GAPDH. [I] Effect of AICAR on Fet-A expression in scrambled or p38MAPK siRNA transfected cells were determined by expressing Fet-A levels, as a ratio to GAPDH (n = 4). [J] Effect of protein synthesis inhibitors, cycloheximide and puromycin, were compared with anisomycin, also a protein synthesis inhibitor, for effects on Fet-A and phosphorylated p38 MAPK expression (n = 3). Data are shown as Means ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). * Indicates p < 0.05.