FIGURE 1.
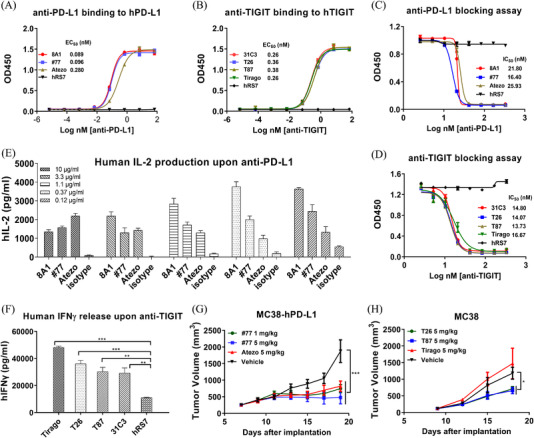
Characterization of anti‐PD‐L1 and anti‐TIGIT mAbs. (A) Anti‐PD‐L1 binding to human PD‐L1‐Fc as detected by ELISA. 8A1, chimeric anti‐PD‐L1; #77, humanized anti‐PD‐L1; Atezo, atezolizumab (Tecentriq) analogue. (B) Anti‐TIGIT binding to human TIGIT‐Fc as determined by ELISA. 31C3, chimeric anti‐TIGIT; T26 and T87, humanized anti‐TIGIT. (C) Anti‐PD‐L1 antibodies blocked the interaction of PD‐L1 with PD‐1 based on ELISA. (D) Anti‐TIGIT antibodies neutralized the binding of TIGIT to PVR based on ELISA. (E) Human IL‐2 production in the Staphylococcal enterotoxin B (SEB)‐activated peripheral blood mononuclear cells (PBMC) assay in the presence of anti‐PD‐L1 antibodies. (F) Human IFNγ production in primary T cells stimulated with OKT3 and anti‐CD28 in the presence of anti‐TIGIT antibodies. hRS7, a humanized IgG1 mAb against Trop 2, was used as a negative control. (G) MC38‐hPD‐L1 tumour cells in C57BL/6‐HU‐PD‐L1 transgenic mice. Anti‐PD‐L1‐hu#77 was administered i.p. at 1 and 5 mg/kg, and atezolizumab analogue at 5 mg/kg every 3 days. Tumour volume was monitored using electronic callipers every other day. (H) MC38 tumour cells inoculated in C57BL/6‐HU‐TIGIT transgenic mice. Anti‐TIGIT was injected i.p. at 5 mg/kg and tumour size was measured every 3 days. Tumour volumes were presented as mean ± SEM. Statistical significance (p‐value) was determined using one‐way or two‐way ANOVA with Tukey's multiple comparison test (*p < .05, **p < .01, ***p < .001)
