Figure 1.
VHH E4-1 binds to the MTBD of Tau
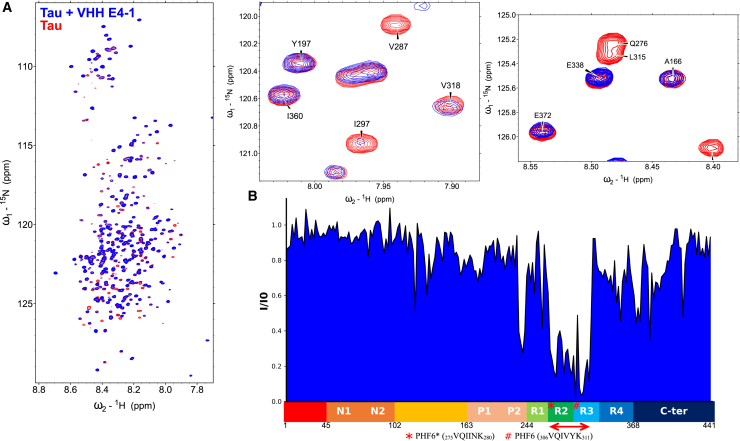
(A) Overlay of 1H, 15N HSQC two-dimensional full spectra and enlargements of free full-length Tau2N4R (in red) or Tau2N4R mixed with non-labeled VHH E4-1 (in blue) (n = 1). In the spectrum of Tau in the presence of VHH E4-1, multiple resonances are broadened beyond detection compared with the Tau control spectrum. (B) Normalized NMR intensities (I/I0) along the Tau sequence with (I0) and (I) corresponding to the resonance intensity when Tau is free in solution or mixed with equimolar quantity of VHH E4-1 (I), respectively. The normalized intensity ratios (I/I0) plot allowed the identification of the Tau MTBD domain as the target of VHH E4-1 interaction. A red double-arrow indicates the region containing the corresponding major broadened resonances, which was mapped to the R2-R3 repeats in the MTBD. N1 and N2 are two inserted sequences in the N-terminal domain (1–163) that are not present in all Tau isoforms (named Tau 0N, Tau 1N or Tau 2N), the proline-rich domain is subdivided in P1 and P2 regions, the MTBD consists of four partially repeated regions, R1 to R4 (in Tau 4R). The R2 repeat is not present in Tau 3R. C-ter is for the C-terminal domain.