Optimization of reaction conditions of 1a with NH2OH·HCl to access 4,5-disubstituted isoxazoles regioisomers 2a and 3a regioselectivelya.
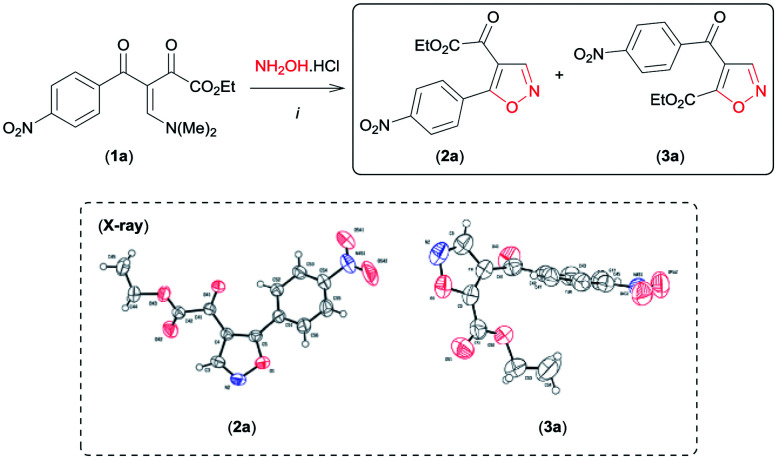
| ||||||
|---|---|---|---|---|---|---|
| Entry | i | Ratiob (%) | Yieldc (%) | |||
| Solvent | Base | Time (h)/Temp. (°C) | 2a | 3a | ||
| 1 | EtOH | — | 10/25 | 35 | 65 | 73 |
| 2 | MeCN | — | 16/25 | 65 | 35 | 81 |
| 3 | EtOH/H2O | — | 10/25 | 40 | 60 | 68 |
| 4 | EtOH | Py | 2/25 | 64 | 36 | 71 |
| 5 | MeCN | Py | 2/25 | 76 | 24 | 87 |
| 6 | MeCN | DBU | 2/25 | —d | — | |
| 7 | MeCN | K2CO3 | 2/25 | —d | — | |
| 8 | EtOH | — | 1/reflux | 23 | 77 | 76 |
| 9 | MeCN | — | 3/reflux | 54 | 46 | 78 |
| 10 | MeCN | Py | 1/reflux | 45 | 55 | 80 |
| 11 | EtOH | Py | 1/reflux | 62 | 38 | 74 |
Reaction conditions: 1a (0.5 mmol), NH2OH·HCl (0.6 mmol, 1.2 equiv.), base (0.6 mmol, 1.2 equiv.), solvent (4 mL).
Calculated from the 1H-NMR spectrum of crude product.
Isolated yield (regioisomeric mixture).
2a and 3a as intractable mixtures of several products.
