Optimization of reaction conditions of 1a with NH2OH·HCl mediated by BF3·OEt2 to access 3,4-disubstituted isoxazole 4a regioselectivelya.
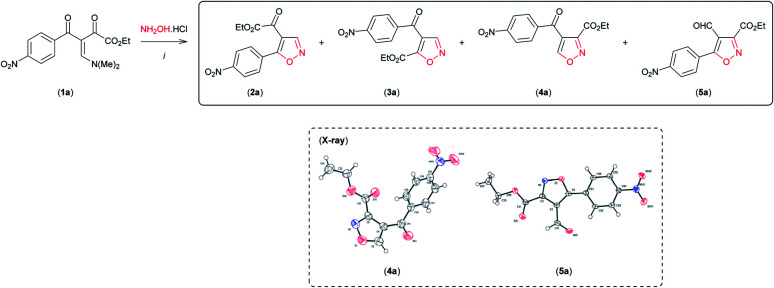
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | i | Ratiob (%) | Yieldc (%) | |||||
| Solvent | BF3·OEt2 (equiv.) | Time (h) | 2a | 3a | 4a | 5a | ||
| 1 | MeCN | 0.5 | 18 | 37 | 13 | 50 | — | — |
| 2 | MeCN | 1.0 | 20 | 22 | 8 | 70 | — | — |
| 3 | MeCN | 1.5 | 24 | 9 | — | 81 | 10 | — |
| 4 | MeCN | 2.0 | 24 | — | — | 90 | 10 | 79 |
| 5 d | MeCN | 2.0 | 5 | — | — | 90 | 10 | 79 |
| 6d | EtOH | 2.0 | 2 | 64 | 36 | — | — | — |
Reaction conditions: 1a (0.5 mmol), NH2OH·HCl (0.6 mmol, 1.2 equiv.), room temperature, solvent (4 mL).
Calculated from the 1H-NMR spectrum of crude product.
Isolated yield (regioisomeric mixture).
Pyridine (1.4 equiv.).
