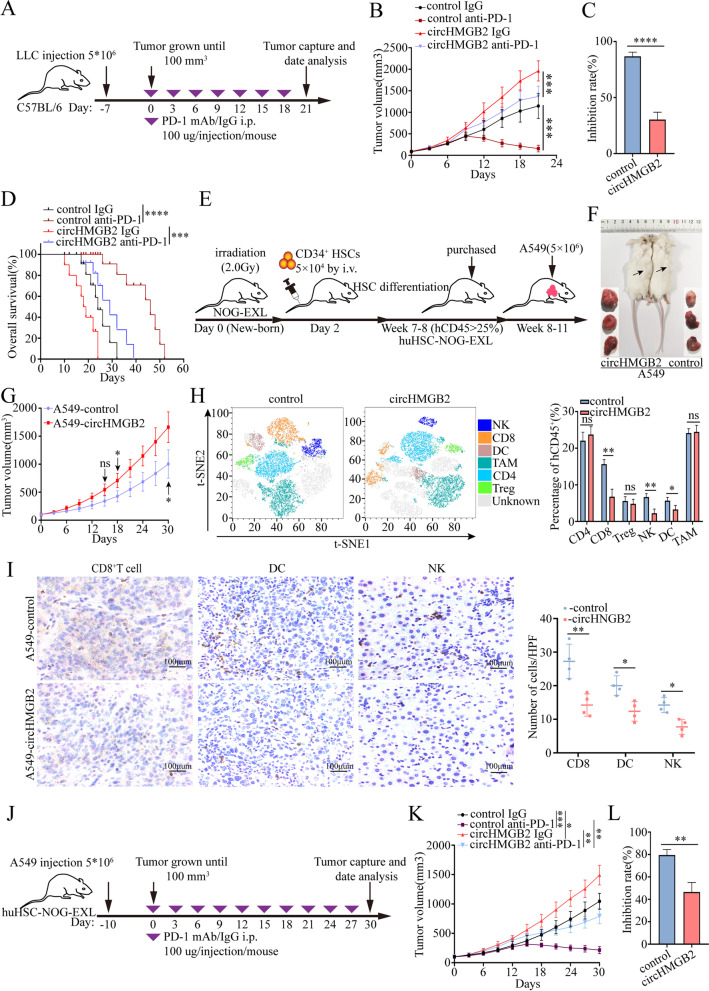
Fig. 4.
CircHMGB2 decreased the efficacy of PD-1 blockade in NSCLC treatment. A A schematic diagram of the treatment plan in C57BL/6 mice. B Growth of subcutaneous tumors in C57BL/6 mice inoculated with LLC-control or LLC-circHMGB2 cells and administered an anti-PD-1 antibody. C The efficacy of PD-1 therapy after the overexpression of circHMGB2. D The prognosis of C57BL/6 mice subcutaneously implanted with LLC-circHMGB2 cells and administered an anti-PD-1 antibody. E A schematic diagram of the establishment of huHSC-NOG-EXL mice. F and G huHSC-NOG-EXL mice were subcutaneously implanted with A549-control and A549-circHMGB2 cells, and the tumor size was assessed every 3 days. H The immune cell profiles of subcutaneous tumors derived from A549-control and A549-circHMGB2 cells were detected by flow cytometry. I IHC staining of CD8+ T cells, DCs, and NK cells in subcutaneous A549-control and A549-circHMGB2 tumors. J A schematic view of the treatment plan in huHSC-NOG-EXL mice. K. Growth of subcutaneous tumors in huHSC-NOG-EXL mice inoculated with A549-control or A549-circHMGB2 cells and administered an anti-PD-1 antibody. L The efficacy of PD-1 therapy after the overexpression of circHMGB2. Data are presented as the means ± SD; n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001, ns: not significant