Standard Gibbs free energies of formation for Cu–Dy intermetallic compounds.
| Intermetallic compound | Formula | T/K | ΔG⊖f/kJ mol−1 |
|---|---|---|---|
| Cu5Dy | ΔG⊖f(Cu5Dy) = −3FΔE4 | 773 | −111.75 ± 1.16 |
| 803 | −109.72 ± 2.61 | ||
| 833 | −109.14 ± 1.45 | ||
| 863 | −108.27 ± 2.03 | ||
| Cu9/2Dy |

|
773 | −108.01 ± 1.19 |
| 803 | −105.89 ± 2.61 | ||
| 833 | −105.23 ± 1.42 | ||
| 863 | −104.42 ± 1.91 | ||
| Cu2Dy |

|
773 | −76.96 ± 1.65 |
| 803 | −75.21 ± 2.45 | ||
| 833 | −74.27 ± 1.92 | ||
| 863 | −73.59 ± 1.81 | ||
| CuDy |
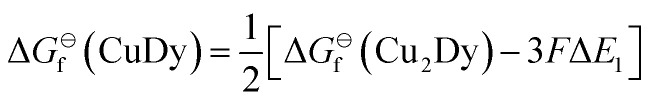
|
773 | −52.95 ± 1.84 |
| 803 | −51.65 ± 2.38 | ||
| 833 | −50.31 ± 1.83 | ||
| 863 | −49.68 ± 1.92 |
