Table 1.
Reductive chlorination of trityl hydrazones.
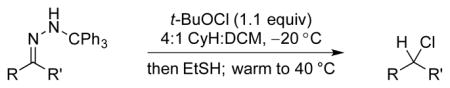
| |||
|---|---|---|---|
| entry | hydrazone | chloride | yield[a] |
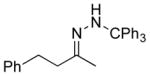
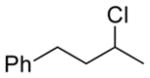
| 1 11a |
|
|
13a 82% |
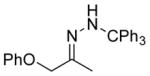
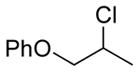
| 2 11b |
|
|
13b 85%[b] |
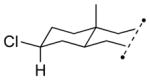
| 3 11c |
|
|
13c 57% (3.4:1) |
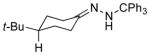
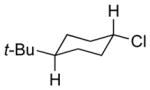
| 4 11d |
|
|
13d 69% (2.9:1) |
| 5 11e |
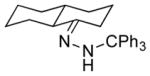
|
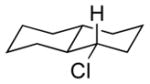
|
13e 71% (1.3:1) |
| 6 11f |
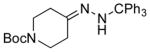
|
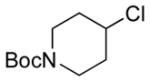
|
13f 56%[b] |
| 7 11g |
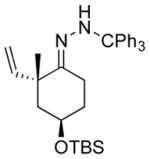
|
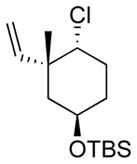
|
13g 50%[c] |
| 8 11h |
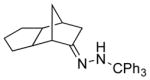
|
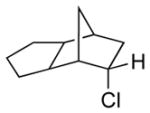
|
13h 83% (21:1) |
| 9 11i |
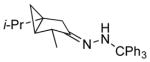
|
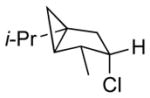
|
13i 70% (2.6:1) |
| 10 11j |
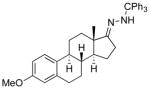
|
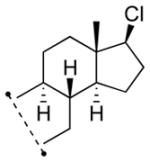
|
13j 67%[d] |
Isolated yields; diastereomeric ratio (dr) determined by 1H NMR of purified chlorides and indicated in parentheses.
NMR yield.
Isolated yield for diastereomer shown (major), 2.8:1 crude dr.
Lithiated hydrazone treated with dichloramine-T.
