The comparison of the current method with previous reports in the arylation of benzoxazole.

| ||||
|---|---|---|---|---|
| Entry | Catalyst | Reagent | Condition | Yield (%) |
| 1 | FeSO4 (0.2 equiv.), H2O/diglyme/O2 |

|
150 °C, 20 h | 70 (ref. 31) |
| 2 | I2 (2 equiv.), PhCl, DMF |

|
130 °C, 30 h | 75 (ref. 30) |
| 3 | [Pd(π-allyl)Cl]2 (0.1 equiv.), PCy3, NaOtBu (2 equiv.), DMF |

|
120 °C, 12 h | 43 (ref. 7) |
| 4 | CuCN(PPh3)2 (10 mol%), PPh3, Cs2CO3, pivalonitrile |

|
Reflux, 24 h | 85 (ref. 28) |
| 5 | Ni(COD)2 (0.1 equiv.), dcype (0.2 equiv.), Cs2CO3 (1.5 equiv.), p-xylene |
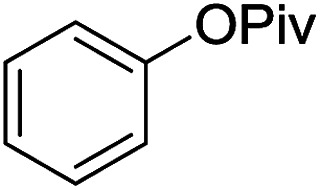
|
140 °C, 22 h | 91 (ref. 29) |
| 6 | Current work: [ZnCl2][ethylene glycol]4 (5 mol%), solvent-free |

|
120 °C, 6 h | 95 |
