Abstract
Microbial blooms that emerge in commercial hatch cabinets consist of apathogenic and pathogenic microorganisms, including Escherichia coli, Enterococcus faecalis, and Aspergillus fumigatus. Objectives of the present study included the development of a multipathogen contamination model to mimic commercial conditions and optimization of sampling methods to quantify bacterial or fungal presence within the hatch cabinet. The pathogen challenge mix (PM) was recreated from select bacterial or fungal isolates recovered from an egg homogenate (EH) derived from the contents of infertile eggs and late embryonic mortalities. Isolates selected for PM included Enterococcus faecalis (∼108 CFU/egg), Staphylococcus aureus (∼107 CFU/egg), Staphylococcus chromogenes (∼107 CFU/egg), Aspergillus fumigatus (∼106 spores/egg), and 2 Escherichia coli (∼108 CFU/egg) isolates. Challenge (100 μL of PM or EH) was administered using a sterile loop to a 28 mm area on the blunt end of the eggshell at day 19 of embryogenesis (DOE). In 3 experiments, microbiological data were collected from environmental hatcher samples (open-agar plate method), fluff samples, postmortem whole-body chick rinse samples, and gastrointestinal tract (GIT) samples to evaluate select bacteria and fungi circulating within the hatch cabinet and colonization of GIT. Cumulative bacterial and fungal recovery from the PM hatching environment from DOE20 to hatch was higher than the nonchallenged group (NC) and EH group at ∼860 and ∼1,730 CFU, respectively. Bacterial recovery from GIT, fluff, and chick rinse samples were similar for the PM and EH group in Exp. 1. However, Aspergillus fumigatus recovery from fluff and chick rinse samples for the PM group was significantly (P < 0.001) higher than the NC and EH group. In Exp. 2 and 3, PM challenge significantly (P < 0.05) increased Gram-negative bacterial recovery from the GIT, fluff and chick rinse samples compared to both the NC and EH group. These data suggest this innovative multispecies environmental contamination model using PM could be utilized to evaluate strategies to mitigate microbial contamination in commercial hatch cabinets in a laboratory setting.
Key words: hatchery, challenge, model, pathogen, broiler
INTRODUCTION
Establishment of a beneficial microbial niche during the neonatal phase is essential to ensure proper development and maturation of the gastrointestinal tract (GIT). In nature, neonatal chicks are exposed to the hen's microbiota. The maternal microbiota is transferred to neonatal chicks within 24 h post-hatch and the direct contact with the hen or maternal microbiota influences the composition of the chick's cecal microbiome (Kubasova et al., 2019). In a commercial setting, there is no physical contact between the hen and chick at hatch. As a result, naive neonates are exposed to a variety of fecal or environmental-derived apathogenic and pathogenic microorganisms during embryonic development and the hatching phase.
The cuticle layer of the eggshell serves as a protective barrier (Board and Halls, 1973), but fluctuation in temperature post-lay may accelerate penetration by certain microorganisms present on the surface of the eggshell (Lock, 1992). Contaminated embryos, or non-viable embryonated broiler chicken eggs, have the potential to explode during incubation due to microbial overgrowth and may harbor pathogens, such as antimicrobial-resistant Escherichia coli and Enterococcus spp. (Karunarathna et al., 2020). If non-viable embryonated eggs are not removed at transfer, the eggs could rupture during late embryogenesis and contaminate the environment and adjacent eggs in the hatch cabinet. As the chicks begin to hatch, any microorganisms that had penetrated the eggshell and effectively replicated within the developing embryo during incubation will be transmitted to the noninfected chicks in the hatch cabinet. Cason et al. (1993) demonstrated that Salmonella contamination, as determined by postmortem whole-body rinses, occurred after the chick had pipped the eggshell. This suggests that, while contamination during embryogenesis may not have occurred, contamination of the chick occurs when the eggshell is breached during pipping. As the chicks begin to hatch, the humidity in the hatching environment rises, boosting microbial proliferation and airborne circulation of microorganisms in the hatching environment (Sheldon and Brake, 1991). These microorganisms serve as pioneer colonizers and are the first to colonize the GIT and other mucosal-associated lymphoid tissues.
Pioneer colonizers influence the enteric microbiota composition and modulate intestinal immune development in broiler chickens (Rubio, 2019). Pioneer colonization by opportunistic pathogens, such as E. coli and E. faecalis, has been associated with elevated flock mortality (Olsen et al., 2012). Additionally, avian pathogenic E. coli (APEC) and E. faecalis have been isolated from the yolk sac of chicks with omphalitis, signifying the presence of both microorganisms at the hatchery level (Walker et al., 2020). However, other potential opportunistic pathogens must be considered. For instance, Staphylococcus aureus infections in chickens have become more common, with most of the strains recovered being genetically similar to S. aureus strains that principally infect humans (Lowder et al., 2009). Additionally, severe S. aureus contamination in the hatchery can induce pneumonia, further validating the need for control at the hatchery level (Smyth and McNamee, 2001; Rodgers et al., 1999). In mice, toxin production by S. aureus prevented elimination by the host immune system, which supported replication of Gram-negative bacteria in the lung and subsequent systemic infection (Cohen et al., 2016). More recently, Wu et al. (2021) demonstrated that S. chromogenes colonization in the upper respiratory tract of chickens promoted infection by Avibacterium paragallinarum, the etiologic agent of infectious coryza. These findings suggest that pioneer colonizers of the upper respiratory tract may facilitate infection and disease caused by opportunistic pathogens. Aside from bacterial infections, fungi such as Aspergillus fumigatus, the primary cause of aspergillosis in poultry, is frequently recovered from commercial hatch cabinets (Thermote, 2006). Aspergillus fumigatus conidia or spores can penetrate the eggshell and replicate in the air cell within the egg, which is inaccessible to any fungicidal compounds applied during the hatching phase (Williams and Brake, 2000). Although colonization by microorganisms during embryogenesis may not always be fatal, the number of microorganisms circulating in the environment will rise as the infected chicks hatch, exposing the naive chicks.
Our laboratory previously developed horizontal challenge models for wild-type and virulent E. coli to model the seeding phenomenon where a small number of contaminated chicks horizontally transmit the challenge at hatch to the noninfected chicks (Graham et al., 2019, 2021; Selby et al., 2021). The horizontal challenge models required in ovo or spray application of E. coli to a small number of the embryos (<10% of the population deemed seeders) at DOE19 to seed the environment and expose the naive contact chicks during the hatching period. We recently demonstrated that exposure to E. coli during the hatching phase increased enteric coliform recovery from naive contact chicks (Graham et al., 2021). The purpose of the current proposed model was to simulate bacterial and fungal contamination in commercial hatch cabinets and evaluate culture-dependent microbiological methods to monitor the microbial load in small-scale hatch cabinets. To reflect environmental exposure that occurs in the presence of exploder eggs or during severe microbial contamination in commercial hatch cabinets, the surface of the eggshell was contaminated.
MATERIALS AND METHODS
Experimental Design
Three experiments were conducted (Exp. 1–3). For each experiment, a total of 2,025 fertile eggs (n = 224–225 per hatcher × 3 hatchers per treatment × 3 treatments) were placed in separately assigned hatcher cabinets. The treatments included: 1) nonchallenged control (NC), 2) egg homogenate (EH) challenge, and 3) pathogen mix (PM) challenge. Hatch cabinets were set up in different rooms within the same building to prevent any potential cross-contamination between cabinets during the hatching phase. The PM challenge consisted of microorganisms recovered from a homogenate prepared from contaminated infertile eggs and late embryonic mortalities removed at transfer at DOE18, including 2 wild-type Escherichia coli isolates, Staphylococcus aureus, Staphylococcus chromogenes, Enterococcus faecalis, and Aspergillus fumigatus. The bacterial isolates were identity-confirmed by 16S sequencing. The fungal isolate was speciated as Aspergillus fumigatus based on colony morphology. Application of challenge in hatchers was conducted by applying 100 µL of the EH challenge (homogenate from non-viable eggs) or PM challenge (amplified species recovered from EH) to the blunt end of the egg's surface. At DOE19 (9:30 am), eggs were briefly removed from the hatch cabinets and the material was distributed over a 28 mm area, or ∼half the size of the air cell, using a sterile disposable loop, simulating the “exploder” phenomenon that occurs commercially. To ensure viability of the challenge material after application, 50% of EH prepared from infertile eggs was resuspended in 50% 2X tryptic soy broth (TSB, cat. no. 90000-378, VWR, Suwanee, GA) supplemented with 0.01% xanthan gum. The PM challenge material was resuspended in 2X TSB supplemented with 0.01% xanthan gum to obtain the desired CFU/egg (for bacterial species) or conidia or spores/egg (Aspergillus fumigatus). This particular vehicle of 2X TSB supplemented with 0.01% xanthan gum has been previously evaluated and does not alter the viability of the challenge organisms or affect chick hatchability (unpublished data). The NC did not receive any treatment. Following hatch, percent hatchability was recorded, and a composite sample of the chick fluff (∼1 g) was collected from the hatching environment. Chicks were immediately euthanized, and samples were collected, including body rinse for surface bacteria and fungi and GIT collected for enumeration of relevant enteric pathogens. Each sample was homogenized with sterile saline by stomaching, 10-fold serially diluted, and plated onto different selective agar plates to enumerate population changes of various bacteria or fungi present by treatment as described below. Hatch cabinet components were thoroughly disinfected, allowed to dry, and then fumigated with formaldehyde between each experiment.
Challenge Preparation
Bacterial Isolates
To prepare the PM challenge for each experiment, 1 mL of each E. coli isolate, S. aureus, S. chromogenes, or E. faecalis was removed from a frozen aliquot and added to 100 mL of tryptic soy broth. The cultures were incubated aerobically at 37°C for ∼18 h. Each Staphylococcus spp. culture was placed on an orbital shaker during incubation, whereas the E. coli and E. faecalis cultures were incubated statically. Postincubation, bacterial cells were washed three times with sterile saline (0.9% NaCl) by centrifugation at 1,800 × g for 15 m. Colony-forming units (CFU) were determined by serial dilution and plating on respective agar media to determine the stock concentration. Cells were then held approximately 16 h at 4°C. On the day of challenge, a specific volume of each challenge organism was concentrated by centrifugation based on the target CFU concentration for application. The pelleted bacterial cells and Aspergillus fumigatus spores were combined and resuspended with the vehicle to achieve the predetermined concentration of each organism for the actual challenge. The EH challenge was simply prepared by removing a frozen aliquot of the material recovered from non-viable embryonated eggs and combined 1:1 with the vehicle. Actual CFU/egg or spores/egg for each microorganism, for PM and EH challenge, was confirmed by spread plating in triplicates on the relevant media described below.
Fungal Isolate
From a thawed aliquot, Aspergillus fumigatus was directly swabbed onto Sabouraud dextrose agar (SDA, cat. no. 95021-184, VWR) supplemented with chloramphenicol 50 mg/L. The methods used to recover and enumerate the Aspergillus fumigatus spores were derived from Sala and Burgos (1972) and National Institute of Health standard operating procedures for model for invasive Aspergillosis (Source link). Aspergillus fumigatus spores/100 uL/egg was confirmed using a hemacytometer and spread plating on SDA supplemented with chloramphenicol 50 mg/mL.
Enumeration of Bacteria and Fungi
Environmental Sampling
The open-agar plate method (Berrang et al., 1995; Kim and Kim, 2010; Graham et al., 2018) was used to enumerate select airborne microorganisms circulating in the hatching environment. For each media used, three agar plates (with the lids removed) were placed open side up on the top tray of the hatchers (G.Q.F. 1550 Digital Cabinet Egg Incubator) using a modified sample port as previously described (Graham et al., 2021) to evaluate Gram-negative bacteria (MacConkey agar, cat. no. 89429-342, VWR), Staphylococcus spp. (mannitol salt agar, MSA agar, cat. no. 89405-680, VWR), Enterococcus spp. (Chromagar Orientation, CO agar, RT412, DRG International, Springfield, NJ), or Aspergillus fumigatus presence in the hatching environment. The open agar plates were placed in the hatch cabinet environment for either 1 m (minute) or 5 m durations based on the media type. A 5-m sampling duration was selected for MacConkey agar (Graham et al., 2021) and for SDA based off of preliminary data (results not shown). However, CO agar, MSA agar, tryptic soy agar (TSA, cat. no. 90002-700, VWR) plates were placed in the hatching environment for 1m at each sampling time point. The hatch cabinet environment was sampled at four time points during the hatching phase: DOE20 8:00 am (∼20% hatch), DOE20 2:00 pm (∼50% hatch), DOE20 5:00 pm (∼80% hatch), and DOE21/DOH 7:00 am (∼100% hatch, DOH). Post-sampling, the agar plates were incubated aerobically at 37°C for 18 h to enumerate total aerobic bacteria, Gram- negative bacteria, and Enterococcus spp. However, select agar plates were incubated for 48 h to determine Staphylococcus spp. and Aspergillus fumigatus presence in the hatching cabinets.
GIT Sampling
For all experiments, the GIT samples (n = 5 chicks/hatcher, n = 15 chicks/treatment) were aseptically removed from chicks after the whole-body rinse samples were collected. The GIT (ventriculus to the cecum) was collected into sterile bags. GIT samples were weighed and homogenized, and 1:4 wt/vol dilutions were made using sterile 0.9% saline. Ten-fold dilutions of each sample from each group were made in sterile 96-well Bacti flat-bottom plates. The diluted samples were plated to evaluate Gram-negative bacteria, Staphylococcus spp., and Enterococcus spp. on the media described above. All plates were incubated aerobically at 37°C. MacConkey and CO agar plates were incubated at 37°C for 18 h. MSA plates were incubated for 48 h. Bacterial counts were expressed as Log10 CFU/g of sample.
Fluff and Chick Rinse Sampling
At hatch, ∼1 g of fluff was collected from each hatch cabinet (n = 1 composite sample/hatch, n = 3 samples/treatment). Gloves were changed between each hatch cabinet during the collection process and eggshell fragments were avoided. Fluff samples were weighed, diluted with sterile 0.9% saline at a 1:50 w/v dilution, and homogenized prior to drop plating samples onto MacConkey agar, TSA, CO agar, MSA agar, and SDA plates. The chick rinse samples were collected postmortem, where five chicks per hatcher (n = 15 per treatment) were placed in a sterile sample bag with 50 mL of sterile saline. The exterior of the chick was gently massaged with the sterile saline for 30 s, as previously described (Bailey et al., 1994). Samples were drop plated as described above to enumerate select microorganisms present on the surface of the chick.
Animal Source
For all experiments, 18-day-old Ross 308 broiler chicken embryos were candled, randomly allocated, and placed into separate hatchers based on treatment group. For all experiments, the surface of the eggshell was not disinfected. All experiments and animal handling procedures complied with the Institutional Animal Care and Use Committee at the University of Arkansas, protocol #20017.
Statistical Analysis
Hatchability and microbial recovery (GIT, fluff, chick rinse) data were subjected to analysis of variance using JMP Pro 13 (SAS, 2016). In Table 3, the air sampling data obtained using the open-agar plate method were reported as an average of 3 agar plates per media for each collection time point and experiment. GIT, fluff, and chick rinse means for select bacterial and fungal recovery were further separated using Tukey's multiple range test (Table 4, Table 5, Table 6).
Table 3.
Microbial recovery (CFU/plate) from the hatching environment at DOE20 (∼20%, ∼50%, or ∼80% hatch) or at DOH immediately prior to hatch pull (Exp 1-3).
| Gram-negative bacteria |
Staphylococcus aureus |
Enterococcus spp. |
Total aerobic bacteria |
Aspergillus fumigatus |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Trt1 | 20%2 | 50% | 80% | DOH | 20% | 50% | 80% | DOH | 20% | 50% | 80% | DOH | 20% | 50% | 80% | DOH | 20% | 50% | 80% | DOH |
| Exp 1 | NC | 1 | 39 | 15 | 37 | 3 | 24 | 18 | 24 | 9 | 18 | 16 | 32 | 18 | 78 | 48 | 74 | 3 | 3 | 2 | 2 |
| EH | 1 | 24 | 43 | 54 | 2 | 158 | 173 | 149 | 56 | 107 | 128 | 291 | 50 | 294 | 542 | 424 | 2 | 3 | 3 | 2 | |
| PM | 43 | 35 | 85 | 46 | 25 | 126 | 90 | 96 | 132 | 121 | 455 | 351 | 124 | 270 | 415 | 431 | 25 | 56 | 66 | 100 | |
| Exp 2 | NC | 0 | 0 | 0 | 1 | 74 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 3 | 3 | 2 | 6 | 14 | 5 | 4 | 1 |
| EH | 4 | 209 | 13 | 38 | 13 | 24 | 64 | 22 | 22 | 38 | 166 | 229 | 79 | 89 | 268 | 318 | 13 | 13 | 9 | 36 | |
| PM | 18 | 454 | 211 | 495 | 17 | 5 | 18 | 47 | 8 | 37 | 51 | 151 | 15 | 112 | 196 | 154 | 17 | 30 | 39 | 14 | |
| Exp 3 | NC | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 4 | 1 | 0 | 1 | 0 |
| EH | 17 | 38 | 68 | 548 | 0 | 1 | 2 | 25 | 1 | 9 | 20 | 105 | 8 | 20 | 23 | 62 | 0 | 1 | 1 | 1 | |
| PM | 184 | 300 | 784 | 239 | 15 | 50 | 43 | 133 | 46 | 68 | 200 | 117 | 43 | 172 | 467 | 159 | 15 | 24 | 22 | 8 | |
n = 3 replicate agar plates/media were exposed to the hatch cabinet environment for 1 m or 5 m based on the type of media.
Non-mannitol fermenting Staphylococcus spp. data not shown.
Darker shaded areas are related to higher CFU recovery for ease of interpretation.
NC: negative control; EH: egg homogenate; PM: pathogen mix.
CFU reported for ∼20% hatch, ∼50% hatch, ∼80% hatch, or immediately prior to hatch pull at DOH as an average of three replicate plates for each time point. n = 3 replicate hatchers/treatment.
Table 4.
Effect of EH or PM challenge application at DOE19 on select bacterial and fungal recovery from the GIT, fluff, or chick rinse samples at DOH (Exp. 1).
| GIT (Log10 CFU/g) | NC1 | EH | PM | SEM | P-value |
|---|---|---|---|---|---|
| Gram-negative bacteria | 3.06b | 4.62ab | 6.71a | 0.54 | 0.0185 |
| Staphylococcus aureus | 0.22b | 1.98a | 2.95a | 0.34 | 0.0025 |
| Staphylococcus spp. (non-mannitol fermenter) | 0.30a | 1.82a | 1.22a | 0.53 | 0.0885 |
| Enterococcus spp. | 0.73b | 6.53a | 5.91a | 0.57 | <0.0001 |
| Fluff (Log10 CFU/g) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 1.34b | 6.03a | 6.79a | 0.57 | <0.0001 |
| Staphylococcus aureus | 0.63b | 6.13a | 7.38a | 0.65 | <0.0001 |
| Staphylococcus spp. (non-mannitol fermenter) | 2.58b | 7.37a | 4.42ab | 0.61 | 0.0031 |
| Enterococcus spp. | 2.16b | 6.45a | 6.47a | 0.45 | <0.0001 |
| Total aerobic bacteria | 3.50b | 6.89a | 6.66a | 0.37 | <0.0001 |
| Aspergillus fumigatus | 0.41b | 1.34b | 4.92a | 0.46 | <0.0001 |
| Chick Rinse (Log10 CFU/mL) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 0.40b | 3.32a | 2.93a | 0.34 | 0.0002 |
| Staphylococcus aureus | 0b | 4.09a | 4.94a | 0.36 | <0.0001 |
| Staphylococcus spp. (non-mannitol fermenter) | 1.00b | 4.85a | 3.68a | 0.33 | <0.0001 |
| Enterococcus spp. | 2.00b | 5.42a | 4.94a | 0.45 | 0.0021 |
| Total aerobic bacteria | 2.06b | 5.03a | 5.09a | 0.27 | <0.0001 |
| Aspergillus fumigatus | 0.25b | 0b | 2.53a | 0.26 | <0.0001 |
Sample size: For fluff, n = 3 composite samples/hatcher plated on respective media in triplicates, so n = 9/treatment group.
For chick rinse and GIT, n = 5/hatcher or n = 15 samples/treatment group.
Means across rows with different superscripts indicate significance at P < 0.05.
NC: negative control; EH: egg homogenate; PM: pathogen mix.
Table 5.
Effect of EH or PM challenge application at DOE 19 on select bacterial and fungal recovery from the GIT, fluff, or chick rinse samples at DOH (Exp. 2).
| GIT (Log10 CFU/g) | NC1 | EH | PM | SEM | P-value |
|---|---|---|---|---|---|
| Gram-negative bacteria | 0.60b | 1.62b | 6.67a | 0.58 | <0.0001 |
| Presumptive S. aureus | 0a | 0.75a | 0.66a | 0.15 | 0.09 |
| Staphylococcus spp. (non-mannitol fermenter) | 0 | 0 | 0 | - | - |
| Enterococcus spp. | 0b | 4.19a | 3.94a | 0.52 | 0.0004 |
| Fluff (Log10 CFU/g) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 0c | 2.78b | 6.31a | 0.55 | <0.0001 |
| Total aerobic bacteria | 3.10b | 6.51a | 6.62a | 0.41 | <0.0001 |
| Presumptive S. aureus | 0.41c | 3.06b | 5.82a | 0.54 | <0.0001 |
| Staphylococcus spp. (non-mannitol fermenter) | 0b | 5.36a | 0b | 0.53 | <0.0001 |
| Lactic acid bacteria | 3.10b | 5.99a | 5.70ab | 0.37 | 0.0008 |
| Enterococcus spp. | 2.65b | 6.38a | 5.73a | 0.42 | <0.0001 |
| Aspergillus fumigatus | 0.82a | 1.98a | 2.58a | 0.39 | 0.1968 |
| Chick Rinse (Log10 CFU/mL) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 0b | 1.03b | 3.21a | 0.31 | <0.0001 |
| Total aerobic bacteria | 0.20b | 5.20a | 5.20a | 0.37 | <0.0001 |
| Presumptive S. aureus | 0c | 2.27b | 3.83a | 0.31 | <0.0001 |
| Staphylococcus spp. | |||||
| (non-mannitol fermenter) | 0b | 3.59a | 0.20b | 0.28 | <0.0001 |
| Lactic acid bacteria | 0.56b | 4.18a | 3.70a | 0.31 | <0.0001 |
| Enterococcus spp. | 0.54b | 4.05a | 3.97a | 0.31 | <0.0001 |
| Aspergillus fumigatus | 0a | 0.36a | 0.36a | 0.11 | 0.3499 |
For fluff, n = 3 composite samples/hatcher plated on respective media in triplicates, so n = 9/treatment group.
For chick rinse and GIT, n = 5/hatcher or n = 15 samples/treatment group.
Means across rows with different superscripts indicate significance at P < 0.05.
NC: negative control; EH: egg homogenate; PM: pathogen mix. Sample size.
Table 6.
Effect of EH or PM challenge application at DOE 19 on select bacterial and fungal recovery from the GIT, fluff, or chick rinse samples at DOH (Exp. 3).
| GIT (Log10 CFU/g) | NC1 | EH | PM | SEM | P-value |
|---|---|---|---|---|---|
| Gram-negative bacteria | 0c | 3.34b | 6.29a | 0.57 | <0.0001 |
| Staphylococcus aureus | 0b | 0.18b | 1.14a | 0.15 | 0.0035 |
| Staphylococcus spp. (non-mannitol fermenter) | 0 | 0 | 0 | - | - |
| Enterococcus spp. | 0b | 3.19a | 4.82a | 0.53 | 0.0003 |
| Fluff (Log10 CFU/g) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 0c | 4.37b | 6.64a | 0.63 | <0.0001 |
| Staphylococcus aureus | 0.93b | 4.61a | 6.10a | 0.51 | <0.0001 |
| Staphylococcus spp. (non-mannitol fermenter) | 0 | 0 | 0 | - | - |
| Enterococcus spp. | 0.41b | 5.92a | 6.25a | 0.54 | <0.0001 |
| Total aerobic bacteria | 1.49b | 6.53a | 7.16a | 0.54 | <0.0001 |
| Aspergillus fumigatus | 0b | 0.41b | 3.73a | 0.38 | <0.0001 |
| Chick Rinse (Log10 CFU/mL) | NC | EH | PM | SEM | P-value |
| Gram-negative bacteria | 0c | 2.25b | 3.51a | 0.29 | <0.0001 |
| Staphylococcus aureus | 0c | 0.93b | 3.43a | 0.26 | <0.0001 |
| Staphylococcus spp. (non-mannitol fermenter) | 0a | 0.40a | 0a | 0.09 | 0.1287 |
| Enterococcus spp. | 0b | 2.67a | 3.61a | 0.29 | <0.0001 |
| Total aerobic bacteria | 0c | 3.38b | 4.91a | 0.33 | <0.0001 |
| Aspergillus fumigatus | 0a | 0a | 0.40a | 0.07 | 0.3499 |
Sample size: For fluff, n = 3 composite samples/hatcher plated on respective media in triplicates, so n = 9/treatment group.
For chick rinse and GIT, n = 5/hatcher or n = 15 samples/treatment group.
Means across rows with different superscripts indicate significance at P < 0.05.
NC: negative control; EH: egg homogenate; PM: pathogen mix.
RESULTS
Hatchability
In all experiments, the application of 100 uL of EH or PM challenge to a 28 mm surface on the blunt end of the eggshell at DOE 19 did not impact hatchability (Table 2). There were no significant (P > 0.05) differences in hatchability across treatment groups by experiment (Table 2).
Table 2.
Percent hatchability (Exp. 1–3).
| Treatment1 | Exp 1 | Exp 2 | Exp 3 |
|---|---|---|---|
| NC | 97.70 ± 0.007 | 96.70 ± 0.007 | 97.70 ± 0.009 |
| EH | 98.00 ± 0.015 | 97.00 ± 0.010 | 97.70 ± 0.003 |
| PM | 98.30 ± 0.003 | 98.30 ± 0.012 | 98.70 ± 0.003 |
| P-value | 0.893 | 0.489 | 0.422 |
n = 3 hatchers/treatment, n = 225/hatcher.
Note: Exp 3 EH n = 224 for one replicate hatcher.
Data reported as mean percent hatchability ± standard error.
NC: negative control; EH: egg homogenate; PM: pathogen mix.
Bacteria and Fungi Recovered From the Hatching Environment (DOE20-DOH)
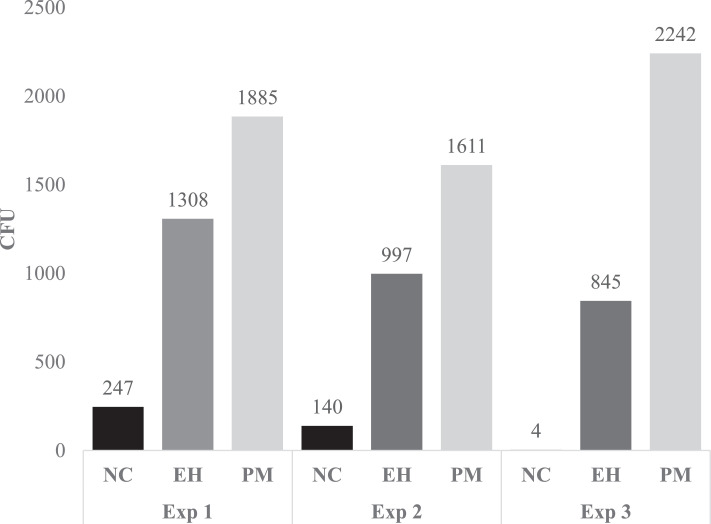
There were 4 collection time points, as described above and in Table 3. The time of sampling was held constant across experiments. Select bacterial and fungal recovery for each time point and experiment is shown in Table 3, and cumulative microbial recovery by treatment and experiment is present in Figure 1. In Exp. 1, there was low-level contamination associated with the embryo source indicated by the increased bacterial recovery (>100 CFU) from the NC hatching environment at all-time points. However, DOE18 embryonated eggs were randomized prior to placement in hatch cabinets to account for naturally acquired contamination.
Figure 1.
Cumulative microbial recovery from the hatching environment across four time points (DOE20-DOH) by treatment (Exp 1–3). CFU reported as recovery of Gram-negative bacteria, presumptive Staphylococcus spp., Enterococcus spp., and Aspergillus fumigatus at ∼20% hatch, ∼50% hatch, ∼80% hatch, and immediately prior to hatch pull at DOH by treatment and experiment. Total aerobic bacterial recovery not included.
Application of EH or PM challenge increased the number of select bacteria circulating during the hatching phase as compared to the NC in all experiments. Bacterial and fungal recovery from the hatching environment at DOE20 and prior to hatch pull was higher for the PM challenge group compared to the EH challenge group (Figure 1). Gram-negative bacterial recovery from the hatching environment was increased in Exp. 2 and 3 for both the EH and PM hatchers, although the PM hatchers had a 2.2- to 4.5-fold increase in cumulative circulating coliforms compared to the EH hatchers (Table 3). The reduction in CFU recovered from the EH hatching environment could be attributed to potential fluctuations in humidity in the hatcher rooms/hatch cabinets across experiments or a potential decline in the viability of the EH during storage at −80C. In contrast, the PM challenge was freshly prepared for each experiment. Regarding fungal recovery, the increase in Aspergillus fumigatus recovered from the PM hatchers compared to EH hatchers could be associated with the ∼2 to 3 log difference in Aspergillus fumigatus between the PM and EH challenge treatment that was applied to the eggshell surface at DOE19 (Table 1).
Table 1.
Microbial recovery from EH or PM material (Exp. 1–3).
| EH1 |
PM |
|||||
|---|---|---|---|---|---|---|
| Colony morphology | Exp. 12 | Exp. 2 | Exp. 3 | Exp. 1 | Exp. 2 | Exp. 3 |
| Gram-negative bacteria (lactose fermenter) | 5.00 × 105 | 6.33 × 105 | 2.00 × 105 | 5.00 × 108 | 1.13 × 108 | 1.07 × 108 |
| Gram-negative bacteria (lactose non-fermenter) | 3.67 × 106 | 2.67 × 106 | 3.67 × 106 | 0 | 0 | 0 |
| Enterococcus spp. | 1.57 × 107 | 1.27 × 107 | 1.03 × 107 | 1.00 × 108 | 2.00 × 108 | 4.67 × 107 |
| Staphylococcus aureus (mannitol fermenter) | 2.00 × 106 | 7.67 × 105 | 6.67 × 105 | 3.23 × 107 | 8.67 × 107 | 7.67 × 107 |
| Staphylococcus spp. (mannitol non-fermenter) | 3.00 × 106 | 5.00 × 106 | 3.33 × 106 | 0 | 0 | 0 |
| Total aerobic bacteria | 1.93 × 107 | 2.80 × 108 | 2.33 × 107 | 4.67 × 108 | 2.67 × 108 | 3.33 × 108 |
| Aspergillus fumigatus | 7.00 × 103 | 1.00 × 104 | 4.00 × 103 | 1.00 × 107 | 1.00 × 106 | 1.00 × 106 |
EH: Egg Homogenate; PM: Pathogen Mix.
Dose (CFU or spores/100 uL/egg) reported as an average of three replicate agar plates.
GIT Samples at Hatch
The mean bacterial recovery from the whole GIT (Log10 CFU/g) at hatch by experiment is presented in Table 4, Table 5, Table 6. Gram-negative bacterial recovery from the GIT at hatch was markedly (P < 0.0001) higher for the PM group compared to NC in all experiments and compared to EH for Exp 2 and Exp 3 (Table 4–6). S. aureus recovery from the GIT was significantly increased due to PM or EH challenge compared to NC in Exp. 1 and Exp. 3, P = 0.0025 and P = 0.0035, respectively (Table 4–6). Enteric recovery of non-mannitol fermenting staphylococci was only observed in Exp 1, although there were no significant differences across treatments (Table 4).
Application of EH or PM to the eggshells at DOE19 significantly (P < 0.0004) increased Enterococcus spp. recovery from the GIT at hatch compared to the NC group in all experiments (Table 4, Table 5, Table 6).
Fluff Samples at Hatch
For all experiments, the mean for select bacterial and fungal recovery (Log10 CFU/g) from composite fluff samples collected at hatch is reported in Table 4, Table 5, Table 6. In Exp 1, application of EH or PM to eggshells at DOE19 significantly (P < 0.0001) increased Gram-negative, total aerobic bacteria, S. aureus, and Enterococcus spp. recovery from fluff samples compared to NC in all three trials (Table 4). In Exp. 2 and 3, PM challenge markedly (P < 0.0001) increased Gram-negative bacterial recovery from fluff samples compared to both the EH and NC group (Tables 5 and 6). Recovery of non-mannitol fermenting staphylococci was significantly (P < 0.0001) higher in fluff samples collected from the EH hatchers in only Exp 2 (Table 6). PM application significantly (P < 0.0001) increased Aspergillus fumigatus recovery compared to NC and EH groups in Exp. 1 and Exp. 3 (Tables 4 and 6).
Chick Rinse Samples at Hatch
The mean bacterial and fungal recovery (Log10 CFU/mL) from postmortem chick rinse samples at hatch for Exp. 1–3 is presented in Table 4, Table 5, Table 6. In Exp. 1, challenge with PM or EH significantly (P < 0.0001, except P = 0.0021 for Enterococcus spp. recovery) increased all nonselective and selective bacterial recovery compared to the NC (Table 4). PM challenge markedly (P < 0.0001) increased Aspergillus fumigatus recovery from chick rinse samples compared to groups NC and EH in Exp 1 only (Table 4). In Exp. 2, EH and PM application significantly (P < 0.0001) increased total aerobic bacteria and Enterococcus spp. recovery from chick rinse samples at the time of hatch compared to the NC (Table 5). However, there were no differences in total aerobic bacteria and Enterococcus spp. recovery between the two challenged groups (Table 5). In Exp. 2, S. aureus and Gram-negative bacterial recovery was numerically increased for PM compared to the EH, but there were no differences in Aspergillus fumigatus recovery across treatment groups (Table 5). EH challenge significantly (P < 0.0001) increased non-mannitol fermenting Staphylococcus spp. recovery compared to EH and NC (Table 5). In Exp 3, EH and PM chick rinse samples had statistically (P < 0.0001) more Gram-negative bacteria, total aerobic bacteria, S. aureus, and Enterococcus spp. recovery compared to group C (Table 6). Alternatively, there were no differences in non-mannitol fermenting Staphylococcus spp. or Aspergillus fumigatus recovery across all treatment groups (Table 6). However, PM treatment significantly (P < 0.0001) increased S. aureus, total aerobic bacteria, and Gram-negative bacterial recovery from chick rinse samples compared to the EH treatment (Table 6).
DISCUSSION
Neonatal chicks may be exposed to circulating apathogenic and pathogenic microorganisms during the hatching phase. Certain bacteria, such as Salmonella spp. are capable of penetrating the eggshell post-lay (Berrang et al., 1999). Non-viable embryonated eggs not removed at the time of transfer may explode due to microbial overgrowth within the egg and contaminate the adjacent eggs and environment in the hatch cabinet (Karunarathna et al., 2017). Chicks may become exposed to the contaminated material on the exterior of the eggshell as they begin to pip. Moreover, as the humidity rises during the hatching phase, chicks are further exposed to the plethora of microorganisms that rapidly proliferate in the environment (Thermote, 2006). Since the relative humidity associated with hatching dictates the onset and proliferation of microbial bloom in the hatch cabinet environment (Magwood, 1964), any variation in microbial recovery between experiments in the present study could be attributed to the natural fluctuation in timing of hatch or proliferation of naturally acquired microorganisms.
Nevertheless, the purpose of the present study was to develop a reproducible multipathogen challenge model to mimic the microbial bloom present in commercial hatch cabinets and validate methods to assess the impact of artificial contamination in small-scale hatch cabinets.
Magwood (1964) determined that the microbial load in the hatching environment was most elevated at the time of hatch. In another study, the observed increase in bacterial load was associated with the onset of hatch (pipping) at DOE19 (Sander and Wilson, 1999). Chick fluff accumulates in the environment during hatch and can be used as a proxy to determine the bacterial and fungal load in a hatch cabinet as a feasible and cheap method to monitor hatchery sanitation (Magwood, 1964). Additionally, storage for up to a week did not alter the level of contamination recovered from the fluff samples. Muira et al. (1964) also showed that fluff samples stored at room temperature for four years remained positive for Salmonella (up to 104-6 CFU/g). Fluff sampling and periodic air sampling of the hatch cabinet environment were investigated as methods to assess airborne contamination in a hatch cabinet (Magwood, 1964). Results published by Magwood and Marr (1964) indicate a direct relationship between the level of air contamination, as measured by fluff and air sampling, and surface contamination in commercial hatcheries. Taken together, these findings suggest that some microorganisms may be capable of remaining dormant in organic matter for an extended period of time. Thus, complete removal of debris and disinfection is important to avoid inadvertent contamination of embryonated eggs and the hatch cabinet environment.
Air sampling methods have been used by the commercial poultry industry to assess hatchery sanitation (Berrang et al., 1995; Kim and Kim, 2010; Graham et al., 2018). The open-agar plate method and air sampling machines have been used to determine the microbial load in the hatching environment. Berrang et al. (1995) collected air samples from the hatching environment on DOE20, or approximately 50% hatch, to determine the level of contamination in a commercial hatchery. To enumerate Enterobacteriaceae over a 2 m sampling period, 2 methods were used: 1) a surface air sampling machine (CFU/180L, 2m) or 2) the open agar plate method (CFU, 2 m). The authors attributed to the 0.71 log increase in Enterobacteriaceae recovery when using the air sampling machine to the higher volume of air sampled as compared to the open agar plate method. However, there were no differences between the sampling methods for Salmonella recovery. In the current study, a 5 m sampling duration was deemed sufficient for enumeration of Gram-negative bacteria and SDA for the particular hatch cabinets. The additional media were placed in the hatch cabinet environment for 1m based on preliminary results (data not shown).
Alternative microbiological techniques have been explored to quantify culturable microorganisms from fluff samples collected from hatchery settings. For instance, Warren et al. (2016) collected 25 fluff samples from 25 commercial hatcheries to evaluate bacterial and fungal load using the pour plate method or the Petrifilm technique and observed no meaningful differences between the 2 techniques. However, in the present study, a single composite fluff sample was collected from each hatch cabinet immediately after hatch pull. The samples were serially diluted and drop plated in triplicate to evaluate the effect of EH or PM challenge on the level of bacterial and fungal contamination in fluff samples as compared to the non-challenged control. The plating technique was simple for quantifying select bacterial and fungal in fluff samples collected from small-scale hatch cabinets.
In addition to fluff sampling, bacterial and fungal recovery from whole-body chick rinses was evaluated in the present study. Whole-body chick rinse sampling has been used to assess Salmonella contamination in hatchery settings (Bailey et al., 1994). Although there were no differences observed for Salmonella recovery from egg shells or whole-body chick rinses, there was a strong correlation between the 2 sampling methods (Bailey et al., 1994). Salmonella has also been recovered from air samples collected from the hatching environment and GIT samples of nonchallenged contact chicks (Cason et al., 1994). Cross-contamination can occur between infected and naive, noninfected chicks during the neonatal period. Infecting 5% of the population with 102 CFU of Salmonella Typhimurium at hatch was sufficient to contaminate 56.7% of the noninfected counterparts within the same pen (Byrd et al., 1999). This suggests that low-level contamination at the hatchery level can increase the risk of horizontal transmission of opportunistic pathogens at the flock level. Thus, for the present study, it was important to assess contamination in the hatching environment using multiple methods, including the open-agar plate method, chick rinse sampling, and fluff sampling since both the EH and PM challenge treatments contained multiple microorganisms.
As previously stated, the microbial load in the hatching environment is affected by the composition of the microorganisms present and the relative humidity in the environment. Naturally acquired contamination was observed in Exp. 1 based on the overall bacterial and fungal recovery from samples collected from the NC group. Moreover, S. aureus recovered from the hatching environment (DOE20 to DOH) and GIT at hatch was numerically higher for all treatment groups compared to the other experiments. Even though S. aureus recovery from the hatching environment and GIT declined in Exp. 2 and Exp. 3, S. aureus recovery from fluff and chick rinse samples of the PM group was elevated compared to both the EH and NC group. The PM challenge more consistently increased Gram-negative bacteria recovery from fluff, chick rinse, and GIT samples compared to the NC and EH groups. Furthermore, there were more Gram-negative bacteria recovered from the hatching environment of the PM group compared to the EH and NC groups at ∼20, ∼50, and ∼80% hatch across all experiments. Application of challenge, whether via EH or PM, similarly increased Enterococcus spp. recovery from fluff, chick rinse, or GIT samples in all experiments. As expected, the increased challenge dose of Aspergillus fumigatus for the PM group increased recovery from the hatching environment and fluff samples collected at hatch compared to EH. These data suggest that the recreated PM is the more appropriate multispecies model to reproduce microbial contamination in commercial hatch cabinets in a laboratory setting.
Since it is not practicable to evaluate and compare novel methods to control the microbial bloom in a commercial hatchery, extensive testing in a laboratory setting is generally required before large-scale application. Several methods were evaluated in the present study to assess the effect of eggshell application “exploder” derived bacteria and fungi as a model to simulate the microbial bloom present in commercial hatch cabinets under laboratory conditions. Moreover, challenge models using a singular microorganism do not truly reflect contamination in commercial hatcher settings. The PM described herein contained multiple microorganisms associated with hatchery contamination, and application to eggshells at DOE19 increased the microbial load in small-scale hatch cabinets. Although the pathogenicity of the specific isolates used in these studies was not presently evaluated, the isolates can be used to artificially increase the microbial load in small-scale hatch cabinets. In future studies, the PM model will be utilized to evaluate alternative methods to formaldehyde fumigation to control the microbial load in the hatch cabinet environment and methods to introduce beneficial pioneer colonizers to displace colonization by potential opportunistic pathogens in neonatal broiler chicks.
DISCLOSURES
The authors have no conflicts of interest to report.
REFERENCES
- Bailey J.S., Cox N.A., Berrang M.E. Hatchery-acquired salmonellae in broiler chicks. Poult. Sci. 1994;73:1153–1157. doi: 10.3382/ps.0731153. [DOI] [PubMed] [Google Scholar]
- Berrang M., Cox N., Bailey J. Measuring air-borne microbial contamination of broiler hatching cabinets. J. App. Poult. Res. 1995;4:83–87. [Google Scholar]
- Berrang M.E., Cox N.A., Frank J.F., Buhr R.J. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. J. App. Poult. Res. 1999;8:499–504. [Google Scholar]
- Board R.G., Halls N.A. The cuticle: a barrier to liquid and particle penetration of the shell of the hen's egg. Br. Poult. Sci. 1973;14:69–97. doi: 10.1080/00071667308416040. [DOI] [PubMed] [Google Scholar]
- Byrd J., DeLoach J., Corrier D., Nisbet D., Stanker L. Evaluation of Salmonella serotype distributions from commercial broiler hatcheries and grower houses. Avian. Dis. 1999;43:39–47. [PubMed] [Google Scholar]
- Cason J.A., Bailey J.S., Cox N.A. Location of Salmonella typhimurium during incubation and hatching of inoculated eggs. Poult. Sci. 1993;72:2064–2068. doi: 10.3382/ps.0722064. [DOI] [PubMed] [Google Scholar]
- Cason J., Cox N., Bailey J. Transmission of Salmonella typhimurium during hatching of broiler chicks. Avian. Dis. 1994;38:583–588. [PubMed] [Google Scholar]
- Cohen T.S., Hilliard J.J., Jones-Nelson O., Keller A.E., O'Day T., Tkaczyk C., DiGiandomenico A., Hamilton M., Pelletier M., Wang Q., Diep B.A. Staphylococcus aureus α toxin potentiates opportunistic bacterial lung infections. Sci. Trans. Med. 2016;8:329. doi: 10.1126/scitranslmed.aad9922. [DOI] [PubMed] [Google Scholar]
- Graham, B., C. Selby, L. Graham, K. Teague, G. Tellez-Isaias, B. Hargis, and C. Vuong. 2021. Development of a wild-type Escherichia coli environmental bloom model to evaluate alternatives to formaldehyde fumigation in broiler chicken hatch cabinets. Poult. Sci. 100:100975. [DOI] [PMC free article] [PubMed]
- Graham B., Selby C., Teague K., Graham L., Vuong C., Latorre J., Tellez G., Hargis B. Development of a novel in ovo challenge model for virulent Escherichia coli strains. Poult. Sci. 2019;98:5330–5335. doi: 10.3382/ps/pez321. [DOI] [PubMed] [Google Scholar]
- Graham L., Teague K., Latorre J., Yang Y., Baxter M., Mahaffey B., Hernandez-Velasco X., Bielke L., Hargis B., Tellez G. Use of probiotics as an alternative to formaldehyde fumigation in commercial broiler chicken hatch cabinets. J. App. Poult. Res. 2018;27:371–379. [Google Scholar]
- Karunarathna R., Popowich S., Wawryk M., Chow-Lockerbie B., Ahmed K.A., Yu C., Liu M., Goonewardene K., Gunawardana T., Kurukulasuriya S., Gupta A., Willson P., Ambrose N., Ngeleka M., Gomis S., others Increased incidence of enterococcal infection in nonviable broiler chicken embryos in Western Canadian hatcheries as detected by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Avian. Dis. 2017;61:472–480. doi: 10.1637/11678-052317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Karunarathna R., Ahmed K.A., Liu M., Yu C., Popowich S., Goonewardene K., Gunawardana T., Kurukulasuriya S., Gupta A., Ayalew L.E., Willson P., Ngeleka M., Gomis S., others Non-viable chicken embryos: an overlooked niche harbouring a significant source of multidrug resistant bacteria in the poultry production. Int. J. Vet. Sci. Med. 2020;8:9–17. doi: 10.1080/23144599.2019.1698145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim K.S. Hatchery hygiene evaluation by microbiological examination of hatchery samples. Poult. Sci. 2010;89:1389–1398. doi: 10.3382/ps.2010-00661. [DOI] [PubMed] [Google Scholar]
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., Matiasovicova J., Faldynova M., Pokorna A., Cizek A., Rychlik I., others Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, J. L. 1992. Factors influencing the behaviour of Salmonella spp. in the hen's egg. University of Bath, UK.
- Lowder B.V., Guinane C.M., Ben Zakour N.L., Weinert L.A., Conway-Morris A., Cartwright R.A., Simpson A.J., Rambaut A., Nübel U., Fitzgerald J.R. Recent human- to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19545–19550. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwood S. Studies in hatchery sanitation: 3. The effect of air-borne bacterial populations on contamination of egg and embryo surfaces. Poult. Sci. 1964;43:1567–1572. [Google Scholar]
- Magwood S.E., Marr H. Studies in hatchery sanitation: 2. A simplified method for assessing bacterial populations on surfaces within hatcheries. Poult. Sci. 1964;43:1558–1566. [Google Scholar]
- Miura S., Sato G., Miyamae T. Occurrence and survival of Salmonella organisms in hatcher chick fluff from commercial hatcheries. Avian. Dis. 1964;8:546–554. [Google Scholar]
- Olsen R.H., Christensen H., Bisgaard M. Transmission and genetic diversity of Enterococcus faecalis during hatch of broiler chicks. Vet. Microbiol. 2012;160:214–221. doi: 10.1016/j.vetmic.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Rodgers J.D., McCullagh J.J., McNamee P.T., Smyth J.A., Ball H.J. Comparison of Staphylococcus aureus recovered from personnel in a poultry hatchery and in broiler parent farms with those isolated from skeletal disease in broilers. Vet. Micro. 1999;69:189–198. doi: 10.1016/s0378-1135(99)00112-1. [DOI] [PubMed] [Google Scholar]
- Rubio L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019;98:695–706. doi: 10.3382/ps/pey416. [DOI] [PubMed] [Google Scholar]
- Sala F.J., Burgos J. Simple method for mass production and collection of conidia from Hemispora stellata. App. Micro. 1972;24:504–505. doi: 10.1128/am.24.3.504-505.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J., Wilson J. Effect of hydrogen peroxide disinfection during incubation of chicken eggs on microbial levels and productivity. Avian. Dis. 1999;43:227–233. [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2016. Using JMP® 13. [Google Scholar]
- Selby C.M., Graham B.D., Graham L.E., Teague K.D., Hargis B.M., Tellez-Isaias G., Vuong C.N. Research note: application of an Escherichia coli spray challenge model for neonatal broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B.W., Brake J. Hydrogen peroxide as an alternative hatching egg disinfectant. Poult. Sci. 1991;70:1092–1098. doi: 10.3382/ps.0701092. [DOI] [PubMed] [Google Scholar]
- Smyth J.A., McNamee P.T. Staphylococci, streptococci and enterococci. Poult. Dis. 2001;5:191–199. [Google Scholar]
- Thermote L. Effective hygiene within the hatchery. Int. Hatch. Prac. 2006;20:18–21. [Google Scholar]
- Walker G.K., Suyemoto M.M., Gall S., Chen L., Thakur S., Borst L.B. The role of Enterococcus faecalis during co-infection with avian pathogenic Escherichia coli in avian colibacillosis. Avian Path. 2020;49:589–599. doi: 10.1080/03079457.2020.1796926. [DOI] [PubMed] [Google Scholar]
- Warren C.A., Weber S.K., Crespo R. Comparison of conventional plating methods and PetrifilmTM for the recovery of aerobic bacteria and mold from hatchery fluff samples. J. App. Poult. Res. 2016;25:48–53. [Google Scholar]
- Williams C., Brake J. Evaluation of application methods for control of Aspergillus fumigatus proliferation on the air cell membrane of in ovo injected broiler eggs. Poult. Sci. 2000;79:1531–1535. doi: 10.1093/ps/79.11.1531. [DOI] [PubMed] [Google Scholar]
- Wu Y., Wang Y., Yang H., Li Q., Gong X., Zhang G., Zhu K. Resident bacteria contribute to opportunistic infections of the respiratory tract. PLoS Pathog. 2021;17:3. doi: 10.1371/journal.ppat.1009436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAID. New animal models for invasive aspergillosis (IA): standard operating procedure for murine inhalational pulmonary aspergillosis. NIH-NIAID-N01-AI-30041. Version 1.10. Accessed Aug. 2019. https://www.niaid.nih.gov/sites/default/files/4ratinhalationalpulmonaryaspergillosis.pdf