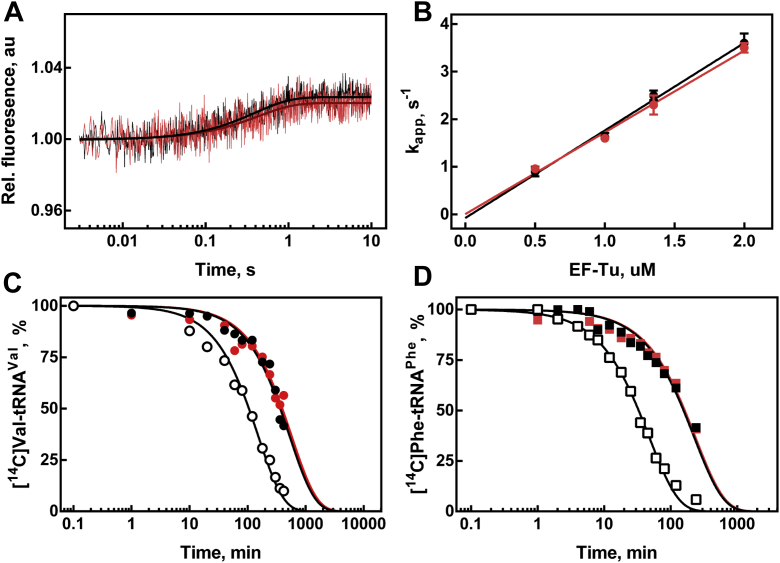
Figure 4.
EF-Tu acetylation does not appear to impact the interaction with aminoacyl-tRNA.A, time courses of ternary complex formation of 0.25 μM Phe-tRNAPhe(Prf16/17) and 1.35 μM EF-Tu·GTP (black trace, black fitting curve) or EF-Tu-Ac·GTP (red trace, dark red fitting curve). B, concentration dependence of ternary complex formation with EF-Tu·GTP (black circles) and EF-Tu-Ac·GTP (red circles). C, portion of unhydrolyzed Val-tRNAVal at specific time points in the presence of EF-Tu·GTP (black circles, kapp = 0.114 ± 0.006 h−1), EF-Tu-Ac·GTP (red circles, kapp = 0.105 ± 0.008 h−1), and in the absence of EF-Tu (open circles, kapp = 0.40 ± 0.02 h−1). D, portion of unhydrolyzed Phe-tRNAPhe at specific time points in the presence of EF-Tu·GTP (black squares, kapp = 0.27 ± 0.02 h−1), EF-Tu-Ac·GTP (red squares, kapp = 0.26 ± 0.02 h−1), and in the absence of EF-Tu (open squares, kapp = 1.27 ± 0.04 h−1). EF-Tu, elongation factor Tu.