Abstract
Cecal epithelial cell damage is a key factor in host injure during the development of E. tenella. The intracellular free Ca2+ of the host cell is closely related to the invasion, development and proliferation of intracellular parasites, and cell damage. To determine the relationship between Ca2+ and host cell damage in the schizogenic stage of E. tenella, we established a chick embryo cecal epithelial cells model of E. tenella infection. Fluorescence staining, flow cytometry, transmission electron microscopy, inhibition and blocking experiments were used to detect the damage effect and mechanism of host cells during the schizogenic stage of E. tenella. The results showed that the host cells cytoskeletal remodeling, cell and organelle structure was destroyed, and apoptosis and necrosis were increased during the schizont stage of E. tenella. Furthermore, the above-mentioned effects of the schizogenic stage of E. tenella on cells can be alleviated by reducing the intracellular Ca2+ concentration in the host cells. These observations indicate that the effect of host cell injury was closely related to Ca2+ during schizont stage of E. tenella.
Key words: Eimeria tenella, Ca2+, cell injury, schizogenic stage
INTRODUCTION
Chicken eimeriosis is a serious global protozoal disease. Eimeria tenella is the most toxic and harmful of the seven species of coccidia found in chickens which mainly affects the caecum (Sandholt et al., 2021). E. tenella sporozoites rapidly develop into schizonts after entering the cecal mucosal epithelial cells. After the schizont matures, a large number of merozoites are released from the cells to damage the cecal epithelial cells, causing the mucosal epithelial cells to undergo rapid degeneration, necrosis, disintegration and shedding (Zhang et al., 2019). In addition, the apoptotic rate of cecal mucosal epithelial cells in infected chickens increased (Xu et al., 2017). Studies have confirmed that the increase of Ca2+ in the cecal mucosa and glandular epithelial cells of chickens can promote the apoptosis of cecal cells after infection with E. tenella (Cui et al., 2016).
Ca2+ plays an important role in maintaining the normal function and structure of cells. It is the most common and important signal transduction component in cells that acts as a second messenger together with cyclic adenosine monophosphate (cAMP) (Lock and Parker, 2020). Studies have shown that Ca2+ plays an important role in causing fatal damage to cells (Sharma et al., 2021). Ca2+ overload is the "last common pathway" for cell damage caused by many reasons. Ca2+ overload can induce degradation of cytoskeleton proteins to destroy the integrity of the connection between the cytoskeleton and the cell membrane; damage to membrane phospholipids makes the bilayer structure disorder and membrane fluidity changes; damage DNA; cause lipid peroxidation of cell membrane; promote the production of oxygen free radicals to aggravate membrane damage (Modesti et al., 2021). Furthermore, Ca2+ plays a crucial role in parasite differentiation, cell scaffold dynamics, migration and colonization and is also a relevant factor in the interaction between the parasite and the host cell (Docampo and Moreno, 2021). The intracellular free Ca2+ of the host cell increases significantly during the process of host cell development of various intracellular parasites, such as Trypanosoma cruzi, Plasmodium falciparum, Leishmania, and Toxoplasma gondii (Docampo and Huang, 2021; Fu et al., 2021; Orrego et al., 2021; Scarpelli et al., 2021). Therefore, the process of intracellular parasites damaging host cells may be closely related to Ca2+ signal transduction.
Chicken cecal epithelial cells are natural host cells of E. tenella (Zhang et al., 2020), but the damage effect of E.tenella on host cells during the schizogenic stage has not been reported. Furthermore, the relationship between Ca2+ and E. tenella-induced cell damage was also unclear. The purpose of this study was to explore the damage effect of E. tenella on host cells during the schizogenic stage and its relationship with Ca2+. Based on the E. tenella-infection, a chick embryo cecal epithelial cells model, fluorescence staining, flow cytometry, transmission electron microscopy, inhibition and blocking experiments, etc. were used to explore the damage and mechanism of E. tenella to host cells during the schizogenic stage. This study reveals the relationship between Ca2+ and host cell damage during the schizogenic stage of E. tenella, and also provide a theoretical basis for the search for anticoccidial drugs that intervene and control the occurrence and development of E. tenella in chickens.
MATERIALS AND METHODS
Ethics Statement
All experiments involving animals were carried out in accordance with institutional animal care and use committee (IACUC) follow the guidelines. And it was approved by the Animal Protection and Utilization Committee of Shanxi Agricultural University, China.
Experimental Animals and Parasite
15-day-old SPF chicken embryos were obtained from Beijing Meri Avigon Laboratory Animal Technology Co., Ltd. (Beijing, China). The E. tenella Shanxi virulent strain (EtSX01) was the strain used in this experiment, which was provided by the Laboratory of Veterinary Pathology in the College of Veterinary Medicine, Shanxi Agricultural University.
Reagents
Thermolysin, Hematoxylin, Eosin, and Trypan Blue were purchased from Sigma (St. Louis, MO); Recombinant murine EGF was purchased from PeproTech (East Windsor, NJ); MTT, Heparin sodium, Paraformaldehyde, 4%, TritonX-100, EGTA were purchased from Solarbio (Beijing, China); Trifluoperazine was purchased from Wuhan Xinhuayuan Technology Development company (Wuhan, China); BAPTA/AM were purchased from ENZO (New York City, NY); FITC-phalloidin was purchased from Cytoskeleton (Denver, CO); ApoDETECT Annexin V-FITC Kit was purchased from Invitrogen (Carlsbad, CA); Mouse monoclonal to beta Actin, Anti-alpha Tubulin antibody, Mouse monoclonal to GAPDH were purchased from abcam(Cambridge, UK); Cy3-conjugated AffifiniPure goat anti-rabbit IgG (H+L) secondary antibodies was purchased from Proteintech (Chicago, IL); bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Scientific (Waltham, MA).
Primary Culture of Chicken Embryo Caecal Epithelial Cells
The chicken embryo caecal epithelial cells were isolated and cultured according to the reported method (Li et al., 2017). Briefly, the cecum was removed from 15-day-old SPF embryos and striped the mesentery carefully; the cecum was fully washed by the PBS buffer, then cut into 1 mm3 size tissue and washed; the tissue was resuspended and mixed by thermolysin (50 mg/L), and digested at 41°C for 2 h; PBS was used to stop the digestion, then centrifuge at 1,200 r/min for 5 min and discard supernatant; the cell pellet was resuspended in 10% FBS low-glycemic DMEM cell culture medium, seeded in a cell culture flask and adhered to the culture for 70 min; the liquid in the flask was collected, centrifuged at 1,200 r/min for 5 min, and then resuspended and counted in DMEM/F12 culture medium containing 2.5% fetal bovine serum (FBS); the cells were seeded in a cell culture plate and could be used for subsequent experiments when the cell attachment rate was reached about 85%.
E. tenella Sporozoite Preparation
The preparation of E. tenella sporozoite was based on the method of Xu et al. (2017). E. tenella sporulated oocysts were centrifuged at 2,000 r/min for 5 min, the supernatant was discarded, and PBS was added to wash thoroughly. The oocysts were suspended in 2 mL PBS and ground with a homogenizer until the excystation rate reached 80%. The above solution was centrifuged at 1,800 r/min for 5 min. Then, precipitate were added suitable quantum spore digestion solution and digest with shaking at 41°C (150 r/min) until 80% of the sporozoites are released. After filtration, the digestion solution was removed by centrifugation at 3,000 r/min for 10 min, and the precipitate was suspended in DMEM solution.
Cytotoxicity Assay
The chicken embryo caecal epithelial cells were plated in 96-well plates at a density of 2 × 104 cells/well in primary cell culture medium containing 2.5% FBS. Then, cells were treated with different inhibitors (8 μmol/L trifluoperazine, 10 μmol/L BAPTA/AM) at the indicated concentrations for 24 h when the cell attachment rate was reached about 85%. After 2 washes with PBS, 15 μL of the MTT solution was added to 100 μL of primary cell culture medium in each well of a 96-well plate and incubated at 41°C for 4 h, then add 150 μL DMSO to each well. The absorbance was measured at 492 nm with a microplate reader.
Host Cell Morphology Determination
The cells were divided into blank control group (C), E. tenella group (T1), trifluoperazine group (T2), BAPTA/AM group (T3) and BAPTA/AM+trifluoperazine group (T4). T2, T3, T4 were incubated respectively with 8 μmol/L trifluoperazine , 10 μmol/L BAPTA/AM or 8 μmol/L trifluoperazine and 10 μmol/L BAPTA/AMfor 4 h. The sporozoites were inoculated into the cells (48-well plate, 1 × 105 sporozoites/well), except for blank control group. Cell slides were taken out at different time points. H&E staining was used to detect the morphological and structural changes of cells. The morphology of 100 cells in each slide was randomly observed and images were captured using a 14.0 MP digital microscope camera.
Cells were grouped and processed as described above. The sporozoites were inoculated into the cells (25 cm2 cell culture flask, 1 million sporozoites/flask), except for blank control group. Cells were taken out at different time points. The cell suspension was centrifuged at 1,500 r/min for 5 min, the supernatant was discarded, and the cell pellet was collected; fixed with 2.5% glutaraldehyde for 2 h; fixed with 1% osmic acid for 3 h; gradient dehydration with 30%, 50%, 70%, 85%, 95%, 100% I, 100% II alcohol; immersion in isoamyl acetate for 20 min; embedding, polymerization, sectioning, and staining. Transmission electron microscopy observes cellular ultrastructure and captures images.
Apoptosis Detection
At indicated times of infection, cells were washed 3 times with PBS, Trypsin-EDTA combined digestion and DMEM containing 10% fetal bovine serum was used to terminate the digestion, and the cells were collected; cells were washed with Binding Buffer; resuspend cells in 500 μL Binding Buffer; 5 µL Annexin V-FITC and 1µL PI were added to samples for 30 min at room temperature; Finally, put it into the flow cytometer for detection. Data analysis with CellQuest software.
Immunofluorescence Staining and Confocal Microscopy
The chicken embryo caecal epithelial cells were plated in 24-well plates with coverslips at a density of 8 × 104 cells/well in primary cell culture medium containing 2.5% FBS. The sporozoites were inoculated into the cells (2 × 105 sporozoites/well). At indicated times of infection, cells were washed with PBS, fixed with 4% paraformaldehyde for 20 min at room temperature, and then permeabilized with 0.2% Triton X-100 for 5 min and blocked with 5% nonfat milk powder for 1 h at 37°C. To stain actin, FITC-phalloidin was added to samples for 30 min at 37°C, hoechst was used to stain the nuclei. To stain tubulin, the cells were incubated overnight at 4°C with anti-alpha Tubulin antibody (1:100). After washing with PBS three times, Cy3-conjugated AffiniPure goat anti-rabbit IgG (H+L) secondary antibody was incubated with PBS at 37°C for 1 h. Hoechst was used to stain the nuclei. After washing with PBS 3 times, the coverslips were mounted onto glass with antifade solution before visualization on a confocal microscope. All images were acquired randomly using a laser scanning confocal fluorescence microscope.
Western Blotting
At indicated times of infection, cells were washed three times with PBS and lysed for 30 min in RIPA buffer (1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride [PMSF] in PBS) on ice. The concentration of protein was determined using a bicinchoninic acid (BCA) protein assay kit. The protein samples (50 mg/lane) were separated using 10% polyacrylamide gels and were transferred to 0.22 μm polyvinylidene fluoride membranes using the Bio-Rad wet transfer system. After blocking for 1 h at 37°C with 5% nonfat dry milk in PBS and reacting with the indicated primary antibodies at 4°C overnight, membranes were exposed to species-specific HRP-conjugated secondary antibodies followed by enhanced chemiluminescence detection by autoradiography. GAPDH was used as a loading control.
Image and Statistical Analyses
Values are presented as the arithmetic mean ± standard error. Each experiment was repeated at least 3 times. The SPSS 17.0 statistical software package (Chicago) was used to perform ANOVA analysis of all data. Histograms were prepared with GraphPad Prism 5.0 software (San Diego, CA). Fluorescence intensity was analyzed by ImageJ software (National Institutes of Health). All results were considered statistically significant at a P value < 0.05.
RESULTS
The Schizogenic Stage of E. tenella Induces Chick Embryo Cecal Epithelial Cells Cytoskeletal Remodeling
The changes in cytoskeleton of chick embryo cecal epithelial cells caused by E. tenella infection were assessed by confocal microscopy. The remodeling rate of microfilament skeleton of host cells inoculated with E. tenella sporozoites was extremely significantly higher than that of the control group from 48 to 96 h, and the remodeling rate increased sequentially. In addition, the microtubule remodeling rate was consistent with the above results (Figure 1A, B). The average expression of actin protein in E. tenella sporozoite host cells was significantly lower than that in the control group from 48 to 96 h. Furthermore, the expression of tubulin significantly lower than that in the control group from 72 to 96 h (Figure 1C). The results indicated that the E. tenella schizogenic stage induced host cytoskeletal remodeling and affected the expression of cytoskeletal proteins.
Figure 1.
The schizogenic stage of E. tenella induces host cells cytoskeletal remodeling. (A) E. tenella infection induces cytoskeletal remodeling. chicken embryo cecal epithelial cells were incubated with E. tenella and fixed at the indicated time points. The untreated cells were used as a control. F-actin was stained with FITC-phalloidin (green) and α-tubulin was stained with anti-α-tubulin (red) and observed by confocal microscopy. Scale bar = 10 μm. (B) Quantitative detection of cytoskeleton changes at different time points after infection. each value represents the average value from 60 to 80 cells from at least 5 regions of 3 representative experiments. (C) β-actin and α-tubulin protein levels in chicken embryo cecal epithelial cells at different infection time points. All of the data are representative of at least three independent experiments. *P < 0.05, **P < 0.01 vs. normal controls.
E. tenella Damages Host Cell and Organelle Structure During Schizogenic Stage
H&E staining of chick embryo cecal epithelial cells showed pale red cytoplasm, clear nuclei, and clear intercellular boundaries (Figure 2Aa). The cells were inoculated with sporozoites for 48 h, and the sporozoites developed into oval trophozoites. The cells shrunken, the intercellular spaces expanded, some cells swelled, vacuoles appeared in the cytoplasm, the chromatin was reduced in basophilia, and the cell density decreased compared with the control group (Figure 2Ab). The cells were inoculated with sporozoites for 72 h, the cell boundary was not obvious, and merozoites of about 3 µm appeared in the cells, which were oval or round in shape (Figure 2Ac). The cells were inoculated with sporozoites for 96 h, the damage to the cell structure is more serious, and the nucleus even disappears (Figure 2Ad). The results indicated that E. tenella schizogenic stage could destroy the morphology of cecal epithelial cells in chick embryos.
Figure 2.
E. tenella damages host cell and organelle structure during schizogenic stage. (A) Host cell changes during the schizogenic stage of E. tenella. The sporozoites were added to chicken embryo caecal epithelial cells and observe under an optical microscope at at different time points. a: normal cells; b: cells infected with E. tenella at 48 h, trophozoites are indicated by black arrows; c: cells infected with E. tenella at 72 h, merozoites are indicated by black arrows; d: cells infected with E. tenella at 96 h (H&E staining, × 400). (B) Changes in host cell organelles during the schizogenic stage of E. tenella. a: normal cells; b: cells infected with E. tenella at 48, 72, 96 h.
Changes of various organelles in host cells observed by transmission electron microscopy during the schizogenic stage of E. tenella. 48 h after inoculation, the microvilli on the cell surface of the host cells become thinner, and then the microvilli shedding decreased, mitochondria swelled, ridges disappeared, vacuolar degeneration, and the rough endoplasmic reticulum degranulated and expanded, and in severe cases burst into Large vacuoles (Figure 2Bb). After 72 h of inoculation, cells detach from adjacent cells, lost microvilli, and develop irregular pseudopodia-like cell protrusions. At the same time, the host cell undergoes different degrees of apoptosis, the cell membrane shrinked and saged, the cytoplasm was divided and surrounded and protrudes outward to “germinate.” The cell body fragments were surrounded by complete cell membranes and contain concentrated cytoplasm, complete organelles or nuclear fragments, etc., forming apoptotic bodies (Figure 2Bc). After 96 h of inoculation, the cell junction structure on the cell membrane adjacent to the cell disappeared, the desmosomes decreased or disappeared, the intercellular connection was lost, and the gap was widened (Figure 2Bd). The results indicated that E. tenella in schizoreproductive stage could severely damage the host cell structure.
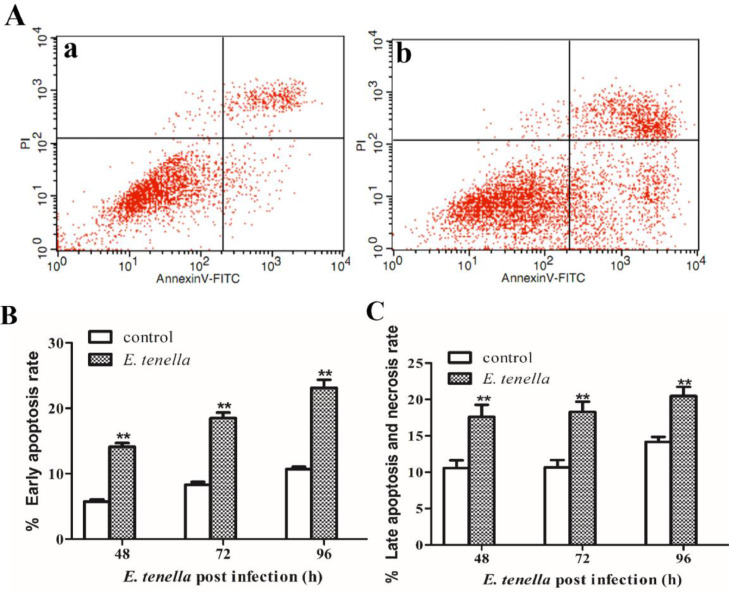
E. tenella in Schizogenic Stage Promotes Apoptosis and Necrosis of Host Cells
The effect of E. tenella schizogenic stage on apoptosis and necrosis was quantitatively determined by flow cytometry. The early, late apoptosis and necrosis rates of host cells inoculated with E.tenella sporozoites were higher than those in the control group from 48 to 96 h, and were most significant at 72 h (Figure 3A). the results are consistent with the above by using CellQuest software to analyze the data at 3 time points (Figure 3B, C). It was confirmed that the E. tenella schizogenic stage can promote the occurrence of host cell apoptosis and necrosis.
Figure 3.
The apoptosis and necrosis of host cells during the schizogenic stage of E. tenella. (A) Apoptosis scatter plot. a: normal cells; b: cells infected with E. tenella at 72 h. (Populations of cells, cell that not undergoing apoptosis were on left bottom panels; cell that undergoing early apoptosis were on right bottom panels; on right top bottom, cell twere in end stage apoptosis or already dead). (B-C) Quantitative detection of early, late apoptosis at different time points after infection. All of the data are representative of at least three independent experiments. *P < 0.05, **P < 0.01 vs. normal controls.
Ca2+ is Closely Related to the Damage of Host Cells During the Schizogenic Stage of E. tenella
To explore the mechanism of E. tenella schizogenic stage to host cell injury, chicken embryonic cecal epithelial cells were treated with an intracellular calcium chelator (BAPTA/AM), a calmodulin inhibitor (trifluoperazine) or BAPTA/AM+trifluoperazine to alter intracellular Ca2+ concentration. First, we tested the effect of chemicals used in this study on cell viability, cell structure, apoptosis rate. The results showed that none of the chemicals used in this experiment had a significant effect on the cell growth rate, cell structure, apoptosis rate (Figure 4).
Figure 4.
Ca2+ channel inhibitors do not affect cell viability. (A) Different inhibitors did not effect on cell growth rate of chicken embryo cecal epithelial cells. (B) Different inhibitors did not effect on cell structure. a: control group; b-d: BAPTA/AM, trifluoperazine, BAPTA/AM+trifluoperazine groups. (C) Quantitative detection of apoptosis in each group. All of the data are representative of at least three independent experiments. *P < 0.05, **P < 0.01 vs. normal controls.
Then, the effects of those drugs on host cell damage during the schizogenic stage of E. tenella were examined. The results showed that BAPTA/AM, trifluoperazine, BAPTA/AM+trifluoperazine all could reduce the cytoskeletal rearrangement, decrease of cytoskeletal proteins, changes in cell morphology and organelle structure, apoptosis and necrosis of host cells caused by E. tenella schizogenic stage, while the effect of BAPTA/AM+trifluoperazine was stronger (Figures 5and 6). The above results indicated that Ca2+ is closely related to the damage of host cells during the schizogenic stage of E. tenella.
Figure 5.
Ca2+ affect cytoskeletal remodeling during the schizogenic stage of E. tenella. (A) Inhibitors attenuate E. tenella infection-induced cytoskeletal remodeling. (B) Quantitative detection of cytoskeletal changes in each group. each value represents the average value from 60 to 80 cells from at least 5 regions of 3 representative experiments. *P < 0.05 vs. controls. (C) β-actin and α-tubulin protein levels in each group. Cells in each group were infected with E. tenella at 72 h. All of the data are representative of at least three independent experiments. *P < 0.05, **P < 0.01 vs. normal controls.
Figure 6.
Ca2+ affect cell injury during the schizogenic stage of E. tenella. (A-C) Changes in host cell and organelle structure and apoptosis in each group. a: E. tenella groups; b-d: BAPTA/AM, trifluoperazine, BAPTA/AM+trifluoperazine groups, respectively. Cells in each group were infected with E. tenella at 72 h. (D) Quantitative detection of apoptosis in each group. All of the data are representative of at least three independent experiments. *P < 0.05, **P < 0.01 vs. normal controls.
DISCUSSION
E. tenella is a typical intracellular parasite. The parasite invades the cecal mucosal epithelial cells and releases merozoites through schizont. E. tenella inhibits host cell apoptosis during pre-infection. But, the cecum will appear microvilli shedding, swelling of mucosal epithelial cells, necrosis and disintegration, etc during the reproduction of merozoites (Zhang et al., 2015). However, the damage effect of E. tenella to cells during the schizogenic stage is not clear. The cytoskeleton plays an important role in all stages of the pathogen's life cycle. Cytoskeleton remodeling often occurs during pathogen development, such as Toxoplasma gondii, Cryptosporidium (Stradal and Schelhaas, 2018). Cytoskeleton remodeling affects cellular protein secretion, signaling and other functions (Jones et al., 2019). In this study, immunofluorescence staining and western blotting were used to observe the changes of cytoskeleton during the schizogenic stage of E. tenella. The results showed that E. tenella caused cytoskeleton remodeling and decreased cytoskeletal protein expression during the schizogenic stage. However, whether this abnormal remodeling and reduced protein expression affect cell function still needs to be further studied.
Intact cells provide cells with a relatively stable internal environment. When cells are damaged, their permeability will change, and intracellular membranous organelles (mainly mitochondria and endoplasmic reticulum) will also be damaged (Wylot et al., 2021). Many extracellular pathogens, for example, C. botulinum, C. tetani; many intracellular pathogens, for example, T. cruzi, L. pneumophila, infection by these pathogens disrupts cell membrane integrity (Welch, 2015). In this study, H&E staining and transmission electron microscopy were used to observe the changes in the morphology and organelles of host cells during the schizogenic stage of E. tenella. The results showed that the host cells were swollen, the cell boundary was not obvious, and the nucleus was lysed during the schizogenic stage of E. tenella. Furthermore, host cell microvilli were shed, mitochondrial ridges disappeared, the rough endoplasmic reticulum was degranulated and enlarged, irregular pseudopodia-like cell protrusions were formed, intercellular junctions were lost, and gaps were widened. It was confirmed that the morphological structure of host cells could be severely damaged during the schizogenic stage of E. tenella.
To survive, parasites interact with host cells and regulate host cell apoptosis through multiple pathways, a process that has been shown to be one of the pathogenic mechanisms of parasites (Kapczuk et al., 2020). Trypanosomes can induce apoptosis in mature CD4+ T cells (Ana et al., 2021). Schistosoma japonicum can promote cell apoptosis by increasing the expression of Fas and FasL in CD4+ and CD8+ T cells (Lundy et al., 2001). When Toxoplasma gondii infected the embryo trophoblast, the expression of Bax and P27 proteins in host cells increased, and the apoptosis rate of host cells increased (Elsalam et al., 2021). After chickens were infected with E. tenella, the concentration of calcium ions and reactive oxygen species in the cecal mucosa and intestinal gland epithelial cells of chickens increased, thereby promoting the apoptosis of cecal cells (Zhang et al., 2015). However, it is not clear whether host cell apoptosis is induced during the schizogenic stage of E. tenella. This study confirmed that the schizogenic stage of E. tenella can cause apoptosis of host cells and increase the necrosis rate. Further confirmation that the schizogenic stage of E. tenella can cause host cell damage.
Mitochondria are the most important calcium reservoirs in cells and are crucial for the regulation of the balance of Ca2+ in the cytoplasm (Alevriadou et al., 2021). Ca2+ plays an important role in maintaining the normal physiological function of cells, which can regulate various types of cell apoptosis (Sukumaran et al., 2021). Under the action of some stimulatory factors, the endoplasmic reticulum releases its stored Ca2+, which are then taken up by mitochondria. Mitochondrial calcium overload leads to its damage, followed by the release of cytochrome c, activation of caspases, and ultimately apoptosis (Marchi et al., 2018). In addition, the schizogenic stage of E. tenella can cause mitochondrial damage was confirmed. In this study, it was shown that changing the intracellular Ca2+ concentration can affect the damage of cells caused by the schizogenic stage of E. tenella. The results confirmed that the schizogenic stage of E. tenella damage host cells are closely related to Ca2+. However, whether this damage is caused by mitochondrial dysfunction through elevated Ca2+ remains to be investigated.
Collectively, our results confirm that E. tenella can severely damage host cells during the schizogenic stage of E. tenella, and this effect is closely related to Ca2+. The results can provide a theoretical basis for a better understanding of the invasion mechanism of E. tenella and the study of new anticoccidial drugs.
Author Contributions
Xiao-ling Lv carried out most of the experiments, wrote the manuscript, and should be considered as primary author. Ming-xue Zheng critically revised the manuscript and the experiment design. Yong-yan Wang, Rui Bai, Li Zhang, Bu-ting Duan, Xuan Lei, Xue-song Zhang, Yong-juan Zhao, Kai-ling Cui, Tong Xu helped with the experiment. All the authors read and approved the final version of the manuscript.
Acknowledgments
This study was funded by a grant from the National Natural Science Foundation of China (Grant No. 31972647), the Applied Basic Research Project of Shanxi Province (Grant No. 201901D211373), the Science and Technology Innovation Fund Project of Shanxi Agricultural University (Grant No. 2020BQ08), the Scientific research project of Shanxi Province outstanding doctoral work award fund (Grant No. SXYBKY2019023), Innovation Projects of College of Veterinary Medicine, Shanxi Agricultural University (Grant No. J202111305).
Disclosures
Xiao-ling Lv carried out most of the experiments, wrote the manuscript, and should be considered as primary author. Ming-xue Zheng critically revised the manuscript and the experiment design. Yong-yan Wang, Rui Bai, Li Zhang, Bu-ting Duan, Xuan Lei, Xue-song Zhang, Yong-juan Zhao, Kai-ling Cui, Tong Xu helped with the experiment. All the authors read and approved the final version of the manuscript.
REFERENCES
- Alevriadou B.R., Patel A., Noble M., Ghosh S., Gohil V.M., Stathopulos P.B., Madesh M. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am. J. Physiol Cell Physiol. 2021;320:C465–C482. doi: 10.1152/ajpcell.00502.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ana Y., Rojas Marquez J.D., Fozzatti L., Baigorri R.E., Marin C., Maletto B.A., Cerban F.M., Radi R., Piacenza L., Stempin C.C. An exacerbated metabolism and mitochondrial reactive oxygen species contribute to mitochondrial alterations and apoptosis in CD4 T cells during the acute phase of Trypanosoma cruzi infection. Free Radic. Biol. Med. 2021;163:268–280. doi: 10.1016/j.freeradbiomed.2020.12.009. [DOI] [PubMed] [Google Scholar]
- Cui X.Z., Zheng M.X., Zhang Y., Liu R.L., Yang S.S., Li S., Xu Z.Y., Bai R., Lv Q.H., Zhao W.L. Calcium homeostasis in mitochondrion-mediated apoptosis of chick embryo cecal epithelial cells induced by Eimeria tenella infection. Res. Vet. Sci. 2016;104:166–173. doi: 10.1016/j.rvsc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Docampo R., Huang G. The IP3 receptor and Ca(2+) signaling in trypanosomes. Biochim. Biophys. Acta. Mol. Cell Res. 2021;1868 doi: 10.1016/j.bbamcr.2021.118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., Moreno S.N. Calcium signaling in intracellular protist parasites. Curr. Opin. Microbiol. 2021;64:33–40. doi: 10.1016/j.mib.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsalam S.A., Mansor A.E., Sarhan M.H., Shalaby A.M., Gobran M.A., Alabiad M.A. Evaluation of apoptosis, proliferation, and adhesion molecule expression in trophoblastic tissue of women with recurrent spontaneous abortion and infected with Toxoplasma gondii. Int. J. Gynecol. Pathol. 2021;40:124–133. doi: 10.1097/PGP.0000000000000683. [DOI] [PubMed] [Google Scholar]
- Fu Y., Brown K.M., Jones N.G., Moreno S.N., Sibley L.D. Toxoplasma bradyzoites exhibit physiological plasticity of calcium and energy stores controlling motility and egress. Elife. 2021;10:e73011. doi: 10.7554/eLife.73011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.C., Zha J., Humphries M.J. Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374 doi: 10.1098/rstb.2018.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczuk P., Kosik-Bogacka D., Kupnicka P., Metryka E., Siminska D., Rogulska K., Skorka M., Gutowska I., Chlubek D., Baranowska-Bosiacka I. The influence of selected gastrointestinal parasites on apoptosis in intestinal epithelial cells. Biomolecules. 2020;10:674. doi: 10.3390/biom10050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng M.X., Xu H.C., Cui X.Z., Zhang Y., Zhang L., Yang S.S., Xu Z.Y., Bai R., Sun X.G. Mitochondrial pathways are involved in Eimeria tenella-induced apoptosis of chick embryo cecal epithelial cells. Parasitol. Res. 2017;116:225–235. doi: 10.1007/s00436-016-5283-z. [DOI] [PubMed] [Google Scholar]
- Lock J.T., Parker I. IP3 mediated global Ca(2+) signals arise through two temporally and spatially distinct modes of Ca(2+) release. Elife. 2020;9:e55008. doi: 10.7554/eLife.55008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy S.K., Lerman S.P., Boros D.L. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL(+) T and B cells during murine Schistosoma mansoni infection. Infect. Immun. 2001;69:271–280. doi: 10.1128/IAI.69.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Modesti L., Danese A., Angela Maria Vitto V., Ramaccini D., Aguiari G., Gafa R., Lanza G., Giorgi C., Pinton P. Mitochondrial Ca(2+) signaling in health, disease and therapy. Cells. 2021;10:1317. doi: 10.3390/cells10061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego P.R., Serrano-Rodriguez M., Cortez M., Araya J.E. In Silico characterization of calcineurin from pathogenic obligate intracellular trypanosomatids: potential new biological roles. Biomolecules. 2021;11:1322. doi: 10.3390/biom11091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholt A.K.S., Xu F., Soderlund R., Lunden A., Troell K., Svard S.G., Wattrang E. Dual RNA-Seq transcriptome analysis of chicken macrophage-like cells (HD11) infected in vitro with Eimeria tenella. Parasitology. 2021;148:712–725. doi: 10.1017/S0031182021000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli P.H., Pecenin M.F., Garcia C.R.S. Intracellular Ca(2+) signaling in protozoan parasites: an overview with a focus on mitochondria. Int. J. Mol. Sci. 2021;22:469. doi: 10.3390/ijms22010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Ramena G.T., Elble R.C. Advances in intracellular calcium signaling reveal untapped targets for cancer therapy. Biomedicines. 2021;9:1077. doi: 10.3390/biomedicines9091077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradal T.E.B., Schelhaas M. Actin dynamics in host-pathogen interaction. FEBS Lett. 2018;592:3658–3669. doi: 10.1002/1873-3468.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P., Nascimento Da Conceicao V., Sun Y., Ahamad N., Saraiva L.R., Selvaraj S., Singh B.B. Calcium signaling regulates autophagy and apoptosis. Cells. 2021;10:2125. doi: 10.3390/cells10082125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.D. Why should cell biologists study microbial pathogens? Mol Biol Cell. 2015;26:4295–4301. doi: 10.1091/mbc.E15-03-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylot M., Whittaker D.T.E., Wren S.A.C., Bothwell J.H., Hughes L., Griffin J.L. Monitoring apoptosis in intact cells by high-resolution magic angle spinning (1) H NMR spectroscopy. NMR Biomed. 2021;34:e4456. doi: 10.1002/nbm.4456. [DOI] [PubMed] [Google Scholar]
- Xu Z.Y., Zheng M.X., Zhang L., Gong X., Xi R., Cui X.Z., Bai R. Dynamic expression of death receptor adapter proteins tradd and fadd in Eimeria tenella-induced host cell apoptosis. Poult. Sci. 2017;96:1438–1444. doi: 10.3382/ps/pew496. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zheng M.X., Xi R., Xu Z.Y., Zhang X.S., Zheng L.L., Bai R., Mi C.L., Hao F.F., Feng Y.P. Comparison of the host cells apoptosis induced by precocious strains and virulent strains of Eimeria tenella. Poult. Sci. 2019;98:4384–4390. doi: 10.3382/ps/pez218. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li S., Zheng M., Zhang L., Bai R., Li R., Hao S., Bai B., Kang H. Effects of the PI3K/Akt signaling pathway on the apoptosis of early host cells infected with Eimeria tenella. Parasitol. Res. 2020;119:2549–2561. doi: 10.1007/s00436-020-06738-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng M.X., Xu Z.Y., Xu H.C., Cui X.Z., Yang S.S., Zhao W.L., Li S., Lv Q.H., Bai R. Relationship between Eimeria tenella development and host cell apoptosis in chickens. Poult. Sci. 2015;94:2970–2979. doi: 10.3382/ps/pev293. [DOI] [PubMed] [Google Scholar]